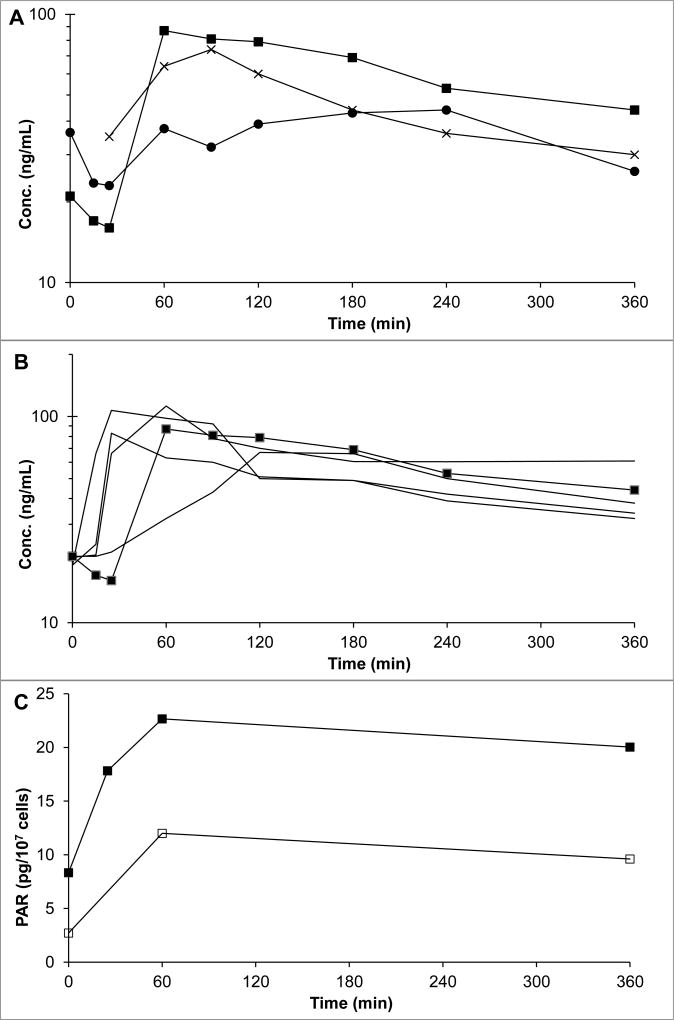
Figure 2.
(A) Plasma (day -2 (X) and day 1 (■)) and ascites (day 1 (●)) concentrations of veliparib in the patient with ascites. (B) Day 1 plasma concentrations of veliparib in the patient with ascites (■) compared to concentrations in five patients without ascites treated at the veliparib dose level. (C) PARP activity in PBMCs (□) and ascites cells (■) in a patient treated with 10 mg veliparib BID and 750 mg/m2 gemcitabine. Time 0 is start of gemcitabine infusion.