Fig. 10.
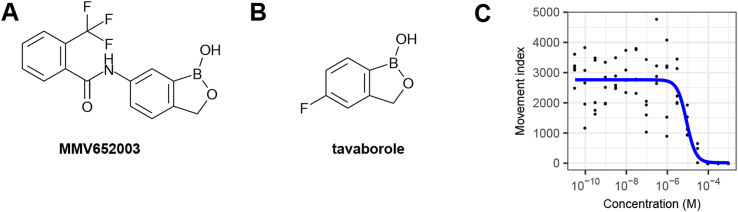
Benzoxaborole compounds as anthelmintics. Structures of (A) the screen hit MMV652003 and (B) the related benzoxaborole tavaborole, which is approved as an anti-fungal. (C) Concentration-response curve for tavaborole in the C. elegans growth assay. N = 5 wells per concentration. Blue line fitted using three parameter log logistic model with drc (Ritz and Streibig, 2005). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)