Abstract
Study Objectives:
Sleep bruxism (SB) is common in children and is associated with somatic symptoms and sleep disturbance. Etiological theories posit the role of anxiety, suggesting youth with anxiety disorders may be at high risk for SB, but empirical data are lacking. Furthermore, parent report rather than polysomnography (PSG) has been used to examine SB-anxiety relationships in children. We examined rates of PSG-detected compared to parent-reported SB in children with generalized anxiety disorder (GAD) and healthy controls. Associations among SB, somatic complaints, and sleep disturbance were also examined.
Methods:
Thirty-one children, aged 7–11 years, completed 1 night of PSG monitoring and 7 daily reports of somatic symptoms. Bruxism events were scored during stage R sleep, stage N1 sleep, and stage N2 sleep.
Results:
Almost one-third of children showed evidence of SB based on PSG. No associations were identified between parent-reported and PSG-detected SB. Rates of SB did not differ between anxious and control groups, though children with GAD showed more tonic bruxisms during stage R sleep. Presence of SB predicted more muscle aches and stomach aches, and children with SB had more awake time after sleep onset than those without bruxism.
Conclusions:
Results indicate poor concordance between PSG-detected and parent-reported SB in children, suggesting that parent report alone is not a reliable method for detection. The lack of association between SB and anxiety status suggests that stress sensitivity rather than anxiety per se may be predictive of SB. Associations between SB, somatic symptoms, and sleep disturbance are congruent with the broader literature.
Citation:
Alfano CA, Bower JL, Meers JM. Polysomnography-detected bruxism in children is associated with somatic complaints but not anxiety. J Clin Sleep Med. 2018;14(1):23–29.
Keywords: anxiety, bruxism, children, polysomnography, sleep, somatic
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research examining sleep bruxism (SB) in children has relied almost exclusively on parent reports that may be biased by certain parent characteristics and/or child behaviors (eg, anxiety). We used both polysomnography (PSG) and parent reports to examine rates of SB in children with an anxiety disorder compared to healthy controls, including associations with daytime somatic symptoms and objective sleep disturbance.
Study Impact: Parent-reported and PSG-identified SB showed no association, raising concern about the reliability of subjective reports. Rates of SB were similar in the anxious and control groups suggesting that temperamental factors other than anxiety may be more closely associated with SB (eg, stress sensitivity). SB was linked with somatic symptoms and sleep disturbance in all youth.
INTRODUCTION
Sleep bruxism (SB) is a sleep-related movement disorder characterized by involuntary and nonfunctional grinding or clenching of the teeth. SB is more common in children than adults1 though prevalence rates in youth range widely, from 13% to 49%.2–5 Because most nighttime bruxing episodes coincide with arousals from sleep,6–8 sleep disruption is a common feature of SB. Further, because the force of bruxing during sleep often exceeds the amplitude of maximum bite force during waking hours, tooth and oral tissue damage are common.9 Other clinical consequences of repeated and sustained mastica-tory muscle activity include temporomandibular joint pain, jaw movement limitation, muscle aches, and chronic headache.10,11
SB is also associated with a range of impairing somatic symptoms. Among adult sufferers, SB is most consistently associated with headaches.12 Although more limited, findings from child studies mirror adult-based outcomes. For example, in one study, SB (detected via parent report) was identified in 29% of children who experienced migraines.13 A linear relationship between migraine frequency and frequency of SB was also found. Another study based on polysomnography (PSG) found an increased prevalence of SB among children with tension headaches specifically.14 Relationships between SB and other common types of somatic complaints in children, such as stomach aches and muscle aches, have been less commonly studied.
Although craniofacial structure and dental irregularities (eg, dental malocclusion) can produce SB, etiological theories consistently highlight the role of psychological factors, and anxiety in particular.15–17 However, findings among adult samples are largely inconsistent. Elevated levels of trait anxiety18,19 and anxiety disorders16 among bruxers have been found in some studies, whereas others have failed to find such differences.20 Another study linked SB with somatic anxiety and muscle tension specifically,17 and still other studies provide evidence of a relationship between anxiety and bruxism during wake but not sleep.21,22
Research examining SB-anxiety relationships in children is more limited but has produced more consistent findings. Several researchers have found significantly higher levels of self- and parent-reported anxiety among children with SB compared to control groups.23–25 Among an adolescent sample, Türkoğlu et al. found significantly higher levels of state anxiety and anxiety sensitivity as well as rates of anxiety disorders among children with SB compared to a matched control group. Generalized anxiety disorder and social phobia were the two most common diagnoses identified.26
An important limitation of previous studies is their reliance on parent report for diagnosing SB. In addition to the common use of nonvalidated questionnaires, parents tend to underestimate SB in their children,27 except perhaps when sleeping in close proximity.2 Other studies have relied on physical evidence of tooth damage among children recruited from dental clinics. Tooth damage is not exclusive to SB, however, and signs of wear may not become apparent until the disorder has been present for several years. Further, investigation of youth recruited directly from dental clinics elevates the possibility of referral/selection bias.28 For these reasons, measurement of muscle activity via submental electromyogram (EMG) as part of overnight PSG monitoring is considered the most reliable indicator of SB.
The current study examined rates of SB in children with generalized anxiety disorder (GAD) compared to matched, typically developing children. None of the participants were recruited specifically for bruxism, dental concerns, or sleep problems. Presence of SB was determined based on standard overnight PSG and concordance with parent reports of SB was examined. We also compared rates of SB based on diagnostic group status, including type of bruxism events and the sleep stage in which SB occurred. Finally, we investigated whether PSG-detected SB was related to daytime somatic complaints and sleep disturbance in all children. Based on previous research we expected to find higher rates of SB in the clinically anxious group and that SB would correspond with greater somatic complaints and wake minutes during the sleep period (ie, sleep disturbance).
METHODS
Participants
The sample included 31 children (14 male) aged 7 to 11 years (mean = 8.87, standard deviation [SD] = 1.45). A total of 14 children met criteria for primary GAD and 17 had no psychiatric diagnoses. Children were recruited via flyers and print advertisements for a study about emotion and behavior at a pediatric hospital in Washington, DC. To be eligible, children had to be fluent in English, live with a parent or caregiver for a minimum of 1 year, and be enrolled in regular education classes. Exclusion criteria included the use of any medications or any illnesses known to affect sleep, current use of treatment services for anxiety or sleep problems, full-scale IQ < 85, a current or lifetime history of psychotic disorder, pervasive developmental disorder, bipolar disorder, eating disorder, substance use, or suicidal ideation/self-harm. Also, because a primary aim of the parent study was to characterize the sleep architecture of children with GAD compared to healthy controls, any child with previously diagnosed or suspected sleep-related breathing disorder (eg, based on parent report of snoring, gasps, or pauses in breathing during sleep) was excluded from participating due to potential confounding effects on sleep architecture. PSG confirmed that no child had a sleep-related breathing disorder.
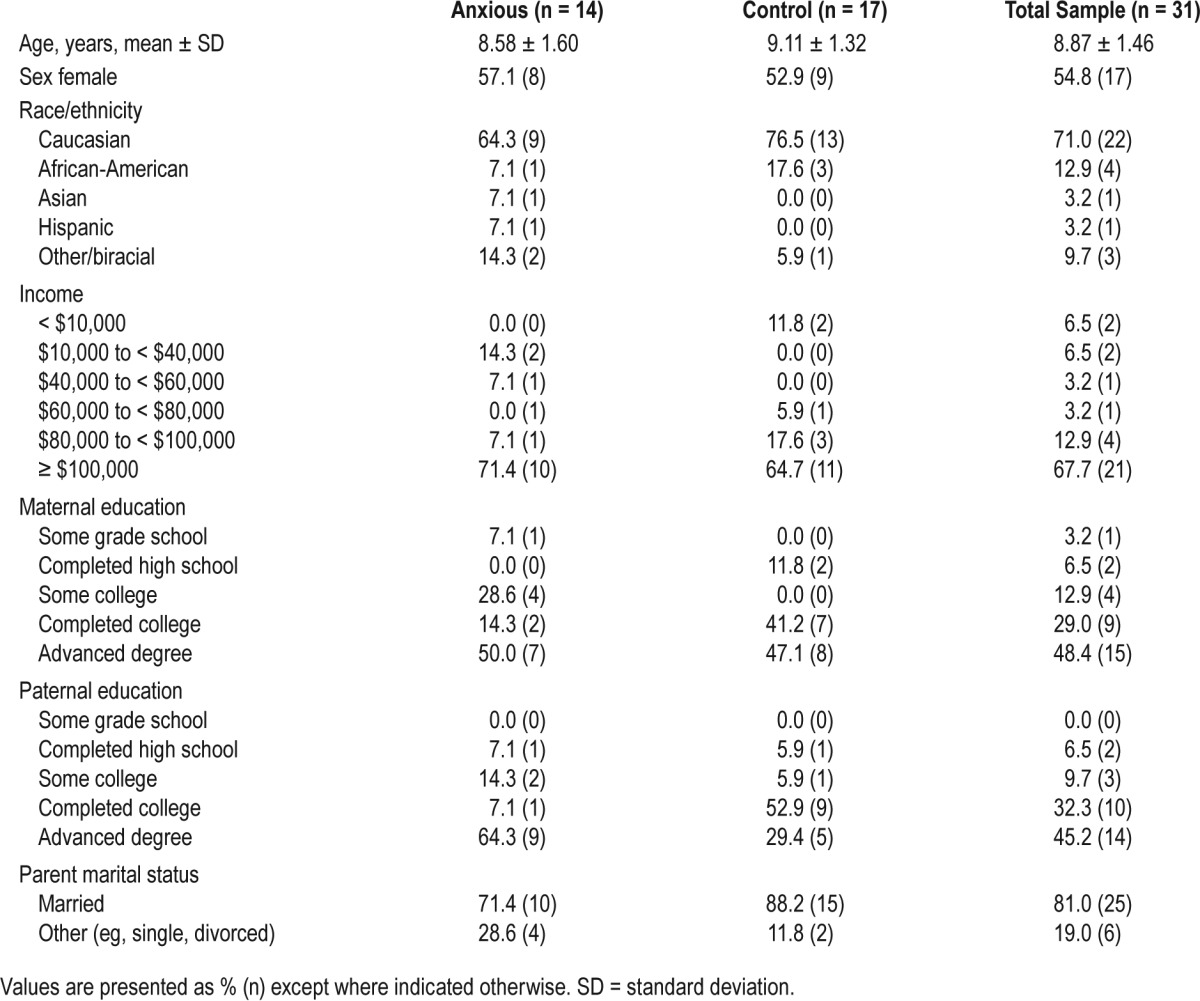
Demographic characteristics for the full sample and both groups are included in Table 1. No significant differences were detected between groups for any demographic variable. Among the clinically anxious sample, comorbid diagnoses included social anxiety disorder (n = 3), attention-deficit/hyper-activity disorder (n = 3), separation anxiety disorder (n = 1), specific phobia (n = 1), depression (n = 1), and oppositional defiant disorder (n = 1).
Table 1.
Group demographics for the full sample, anxious, and control groups.
Study Procedures
All procedures were approved by an Institutional Review Board and parents and children provided consent/assent. Study participation involved an initial diagnostic assessment, 1 week of actigraphy and nightly phone calls, and an overnight PSG. During the diagnostic assessment, children and their parents completed separate semistructured interviews and filled out questionnaires. Afterward, eligible children were given acti-graphs to wear for 1 week. Each evening during the actigraphy week, study staff telephoned families and asked parents and children about anxiety and somatic symptoms experienced that day. Immediately following the 1-week assessment, children completed an overnight PSG in a sleep laboratory, accompanied by their parent or guardian. Actigraphy data were examined to ensure adequate sleep prior to the PSG night. All families were compensated for their time.
Measures
Diagnostic Interview
All children underwent structured diagnostic interviews using the Anxiety Disorders Interview Schedule for DSM-IV - Child and Parent versions (ADIS-C/P).29 The ADIS-C/P assesses a range of clinical symptoms, including anxiety, mood, and externalizing disorders and is considered the gold standard for diagnosing anxiety disorders in children, with high interrater and test-retest reliability.30,31
Daily Symptom Reports
Phone calls were completed daily on 7 consecutive evenings during which the parent and child indicated whether the child experienced any of the following symptoms that day: (1) muscle aches/tension, (2) headaches, and (3) stomach aches. All symptoms were rated on a scale from 0 (none) to 3 (a lot).
Bruxism Measures
CHILD SLEEP HABITS QUESTIONNAIRE (CSHQ): Parents completed the CSHQ, a well-validated scale used to screen for a variety of sleep problems in children.32 The CSHQ includes the item, “Child grinds teeth during sleep” to which parents responded using 3-point scale: 1 (rarely), 2 (sometimes), 3 (usually). SB was considered to be present if parents reported that their child sometimes or usually grinds his or her teeth at night.
POLYSOMNOGRAPHY (PSG): Standard, multichannel PSG was conducted using Medcare amplifiers and Rembrandt version 9.0 Sleep Acquisition Software (Natus Medical Incorporated, Pleasanton, California, United States). Registered polysomnographic sleep technicians (RPSGT) experienced in working with children and scoring pediatric sleep records conducted and scored sleep studies in 30-second epochs based on American Academy of Sleep Medicine (AASM) criteria.33 All studies were conducted and scored under the supervision of a board-certified sleep physician. All technicians were blinded to child diagnostic status. Electroencephalogram (EEG; frontal, central, and occipital regions), electrooculogram, EMG (submental, right/left tibial), electrocardiogram, nasal pressure, thoracic and abdominal respiratory effort, and oximetry data were collected.
PSG-DETECTED BRUXISM: Bruxism events were scored by trained study staff with previous PSG scoring experience and blind to child group status. Scoring was conducted in 30-second epochs in accordance with AASM criteria.33 Bruxism events were scored during stage R sleep, stage N1 sleep, and stage N2 sleep. Powerline filters were set at 60 Hz, with additional high and low pass EMG filters set at 100 Hz and 10 Hz, respectively. Bruxism events were classified as either tonic (sustained EMG burst lasting more than 2 seconds), phasic (3 or more rhythmic EMG bursts of 0.25 to 2 seconds in duration), or mixed (both sustained and rhythmic). Children showing more than 2 bruxism episodes per hour of sleep received a diagnosis of SB.34,35
Statistical Analyses
Normality analyses were conducted on all variables associated with PSG-identified SB (eg, total episodes per hour, episodes per hour during stage N2 sleep, episodes per hour during stage R sleep) and daytime symptoms. The Shapiro-Wilk test indicated that all variables deviated significantly from normality (P < .001), thus nonparametric analyses were conducted. Because overall endorsement of child and parent-reported symptoms during the 7 days of daily phone calls was low (ie, the distribution was positively skewed), daily symptoms were examined as dichotomous variables whereby the symptom was considered to be present if either the child or parent endorsed its presence. Group differences in categorical variables (eg, parent-reported and PSG-detected SB) were examined using chi-square tests, and continuous variables (eg, bruxism episodes per hour) were examined using Mann Whitney U tests. Regression models were conducted to examine whether SB was associated with daytime somatic symptoms.
RESULTS
Missing Data
Three participants were missing data for 2 of the 7 daily phone calls and parent report of SB was missing for 1 child. Three children had some missing data during PSG recordings due to lost EEG or EMG electrodes during the night, though all PSG recordings contained stage N1 sleep, stage N2 sleep, and stage R sleep. Among the 3 children with missing PSG data, 1 showed evidence of SB whereas 2 were considered nonbruxers. Because the presence of SB was detected based on bruxism events per hour of sleep, and given the small sample size, we included partial PSG records in our analysis.
Rates of SB Based on PSG and Parent Reports
According to PSG data, 32.3% of all children (n = 10) showed evidence of SB. By comparison, 26.7% of children (n = 8) were identified to have SB according to parent reports. However, a chi-square test showed no significant association between parent-reported and PSG-detected SB in the full sample. We also examined within-group associations between PSG and parent-detected SB for anxious and healthy children separately. Fisher exact tests showed no association between measurements in either group.
SB in Children With and Without Anxiety Disorders
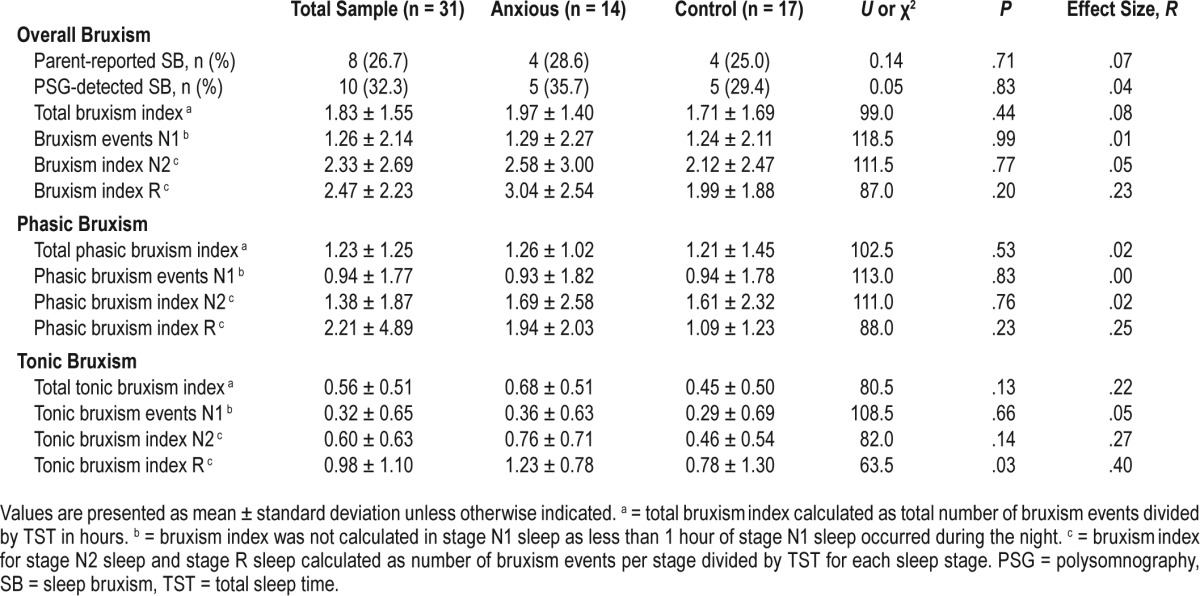
As shown in Table 2, overall rates of SB did not differ between the clinically anxious and control groups based on either PSG or parent reports. Given the exploratory nature of the study, we also examined whether the groups differed in terms of type of bruxism events (tonic or phasic) or the sleep stage (stage N1 sleep, stage N2 sleep, or stage R sleep) in which they occurred. No group differences were detected for tonic or phasic bruxism episodes or for SB episodes detected during stage N1 sleep, stage N2 sleep, or stage R sleep. However, children with GAD showed a significantly greater proportion of tonic bruxism episodes during stage R sleep (U = 63.50, P = .03). No other group differences were detected.
Table 2.
Differences in SB variables based on diagnostic group status.
SB and Somatic Symptoms
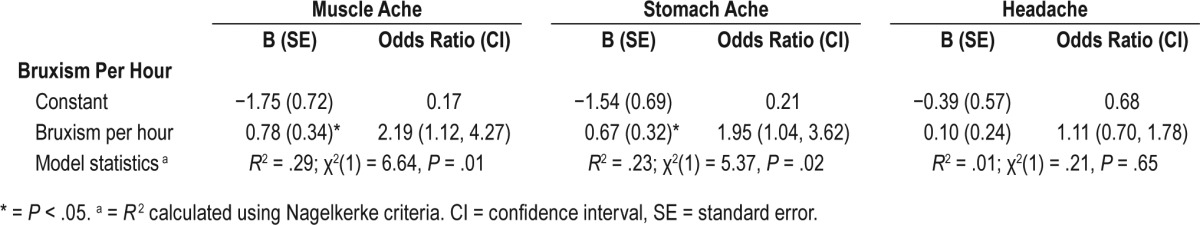
Logistic regression models were used to examine whether SB predicted different types of somatic complaints. Three models were run with number of PSG-detected bruxism episodes per hour as the independent variable and each of the three somatic symptoms (headache, muscle ache, and stomach ache) as dependent variables. Number of bruxism episodes per hour significantly predicted the presence of muscle aches (P = .01) and stomach aches (P = .02) but not headaches in all children (see Table 3).
Table 3.
Sleep bruxism as a predictor of child somatic complaints.
Impact of SB on Sleep
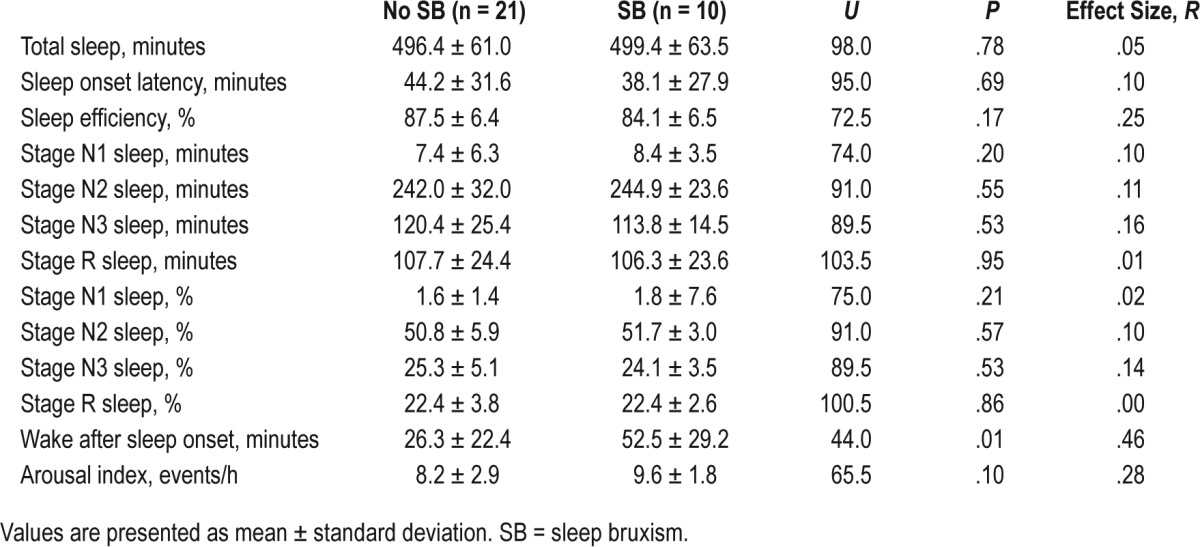
Comparison of PSG sleep variables and architecture between the clinically anxious and control children included in the current study have been reported elsewhere, with no differences found for total sleep time or wake after sleep onset.36 We therefore provide descriptive statistics for sleep variables based on SB status (only) in Table 4. As shown, sleep onset latency, total sleep time, and sleep efficiency did not differ in children with and without PSG-identified SB. Similarly, no differences in sleep stage distribution (stage N1 sleep, stage N2 sleep, or stage R sleep percentage) or arousal index were found based on SB status. However, children in the SB group showed a significantly greater proportion of wake after sleep onset than the control group (Mann-Whitney U = 44.0, P < .01).
Table 4.
Descriptive statistics for sleep variables based on SB status.
DISCUSSION
To our knowledge, this is the first study to use overnight PSG to detect SB in a sample of school-aged children with anxiety disorders and matched typically developing controls. Several interesting findings were noted. First, overall rates of SB found using PSG (32.3%) and parent reports (26.7%) are generally consistent with rates found in several previous studies. At the same time, we found no relationship between the children identified using each of these methods. Because PSG is the most reliable method available for detecting SB, these results suggest that parents incorrectly identify some children as having SB while missing others. Although night-to-night variability in SB might account for reduction in agreement rates, we found no meaningful relationship overall between parent reports and PSG. The clinical and research implications of this finding are significant in suggesting that parent reports alone are inadequate for reliable detection of SB in children.
A number of studies have documented elevated rates of anxiety and anxiety disorders (including GAD) among children with SB,23–26 leading us to expect to find higher rates of SB in our clinical group. However, no differences in SB were detected between clinically anxious and typically developing children based on either PSG or parent reports. Also, because only PSG can provide information about the type and timing of bruxing events, we compared the groups based on type of bruxism episodes (tonic and phasic) and the sleep stage (N2 and R) in which they occurred. Nonsignificant differences were found for all comparisons with the exception of a significantly greater proportion of tonic bruxism episodes during stage R sleep among children with GAD. Although interesting, the reliability of this finding is unclear given the number of comparisons in our study and it awaits replication.
Previous studies linking SB with elevated rates of child anxiety have relied solely on parent reports and/or dental examination. Although our study is unique in its inclusion of children with clinical levels of anxiety, rates of SB among children with GAD and healthy controls did not differ based on either PSG or parent reports. Thus, in line with findings from several adult-based studies,20,37,38 anxiety may be an insensitive predictor of SB in children. Although expensive and unnecessary in most cases, PSG is the only method by which SB can be detected unequivocally.39 A further possible explanation for divergent findings includes the fact that our sample was not recruited from a dental clinic, nor were assessment measures specific to teeth grinding or clenching, both of which might significantly increase the subjective detection of SB.
Another novel finding among our child sample includes a greater incidence of muscle aches and stomach aches among children with SB. Ferreira-Bacci and colleagues24 similarly found elevated levels of somatic symptoms, including stomach aches, among children with SB. However, contrary to a wealth of data in both children and adults, we found no association with headaches in our sample. It bears mentioning that previous findings suggest a link between SB and tension headaches specifically14 which are typically triggered by stress and characterized by bilateral, tightening pain of the scalp or neck.40 Because tension headaches also tend to be milder in pain intensity and shorter in duration than other types of headaches,40 they may have been more easily overlooked during evening phone calls. It is also possible that sensitivity to stress20,37,38 moderates the occurrence of tension headaches in children, which was not examined in the current study.
In line with previous research, children with SB evidenced significantly greater sleep disruption in the form of wake minutes during the sleep period than children without SB. The specific consequences of sleep disruption in this population are currently poorly understood, and the extent to which sleep fragmentation, either alone or in conjunction with sustained masticatory muscle activity, might produce greater physical and mental health problems among children who brux is also not known. Previous research has nonetheless found number of arousals during sleep to correspond with both higher somatic and behavior problems in children with SB,6 suggesting this to be a vital area for future investigations.
Our study has several limitations. First, our sample size was relatively small and our results require replication among larger samples of clinically anxious youth. Children in the anxious group were not receiving any form of treatment, which could be indicative of less severe anxiety than found in other clinically anxious samples. One night of PSG monitoring might have been inadequate to capture SB among children who grind/clench their teeth only occasionally. PSG was also conducted in a novel sleep laboratory environment that could have increased stress and/or anxiety in all children and artificially reduced some of the variability observed between groups. Although we did not utilize video monitoring in the current study, this can be helpful in distinguishing SB from other oro-facial and masticatory movements. We also assessed somatic symptoms during the week leading up to the PSG night, which is less ideal than assessing SB and somatic complaints over the same period.
It also seems important to note that we identified SB based on the amplitude of submental EMG activity relative to background EMG. Although this method is consistent with AASM criteria and previous studies, it may be less sensitive in detecting SB when concern regarding the overall variability of muscle activity exists. For example, individuals with GAD show increased levels of muscle activity and tension during wake,6 which may also be present during the sleep period and complicate detection of SB. Future studies using PSG to investigate SB in this population might therefore consider the use of alternative methods.
In conclusion, reported rates of SB in children vary greatly, with the highest rates identified in studies relying solely on parent reports.1 In addition to the range of factors that can bias subjective assessments, the current lack of validated diagnostic criteria for SB in children undoubtedly contributes to divergent findings in the literature. We found approximately one-third of our sample to have evidence of SB based on PSG, but overall agreement with parent-detected SB was low. Our study also failed to provide evidence of higher rates of SB among a group of clinically anxious compared to typically developing controls (based on either PSG or parent reports). We did identify a greater proportion of tonic bruxism episodes during stage R sleep in the anxious group, but this novel finding awaits replication. Last, consistent with previous research, the presence of SB was associated with greater somatic complaints and sleep disruption in all children. Investigation into whether and how these symptoms might serve as mechanisms for the development of other health problems among children with SB is critical.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This project was funded by grant #K23 MH081188 from the National Institute of Mental Health awarded to the first author. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- ADIS-C/P
Anxiety Disorders Interview Schedule for Children and Parents
- CSHQ
Child Sleep Habits Questionnaire
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EEG
electroencephalogram
- EMG
electromyogram
- GAD
generalized anxiety disorder
- PSG
polysomnography
- SB
sleep bruxism
- TST
total sleep time
REFERENCES
- 1.Machado E, Dal-Fabbro C, Cunali PA, Kaizer OB. Prevalence of sleep bruxism in children: a systematic review. Dental Press J Orthod. 2014;19(6):54–61. doi: 10.1590/2176-9451.19.6.054-061.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheifetz AT, Osganian SK, Allred EN, Needleman HL. Prevalence of bruxism and associated correlates in children as reported by parents. J Dent Child (Chic) 2005;72(2):67–73. [PubMed] [Google Scholar]
- 3.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17(8):739–743. [PubMed] [Google Scholar]
- 4.Serra-Negra JM, Paiva SM, Seabra AP, Dorella C, Lemos BF, Pordeus IA. Prevalence of sleep bruxism in a group of Brazilian schoolchildren. Eur Arch Paediatr Dent. 2010;11(4):192–195. doi: 10.1007/BF03262743. [DOI] [PubMed] [Google Scholar]
- 5.Carra MC, Huynh N, Morton P, et al. Prevalence and risk factors of sleep bruxism and wake-time tooth clenching in a 7- to 17-yr-old population. Eur J Oral Sci. 2011;119(5):386–394. doi: 10.1111/j.1600-0722.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 6.Herrera M, Valencia I, Grant M, Metroka D, Chialastri A, Kothare SV. Bruxism in children: effect on sleep architecture and daytime cognitive performance and behavior. Sleep. 2006;29(9):1143–1148. doi: 10.1093/sleep/29.9.1143. [DOI] [PubMed] [Google Scholar]
- 7.Macaluso GM, Guerra P, Di Giovanni G, Boselli M, Parrino L, Terzano MG. Sleep bruxism is a disorder related to periodic arousals during sleep. J Dent Res. 1998;77(4):565–573. doi: 10.1177/00220345980770040901. [DOI] [PubMed] [Google Scholar]
- 8.Saletu A, Parapatics S, Anderer P, Matejka M, Saletu B. Controlled clinical, polysomnographic and psychometric studies on differences between sleep bruxers and controls and acute effects of clonazepam as compared with placebo. Eur Arch Psychiatry Clin Neurosci. 2010;260(2):163–174. doi: 10.1007/s00406-009-0034-0. [DOI] [PubMed] [Google Scholar]
- 9.Yap AU, Chua AP. Sleep bruxism: Current knowledge and contemporary management. J Conserv Dent. 2016;19(5):383–389. doi: 10.4103/0972-0707.190007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35(7):476–494. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 11.Kampe T, Tagdae T, Bader G, Edman G, Karlsson S. Reported symptoms and clinical findings in a group of subjects with longstanding bruxing behaviour. J Oral Rehabil. 1997;24(8):581–587. doi: 10.1046/j.1365-2842.1997.00540.x. [DOI] [PubMed] [Google Scholar]
- 12.De Luca Canto G, Singh V, Bigal ME, Major PW, Flores-Mir C. Association between tension-type headache and migraine with sleep bruxism: a systematic review. Headache. 2014;54(9):1460–1469. doi: 10.1111/head.12446. [DOI] [PubMed] [Google Scholar]
- 13.Miller VA, Palermo TM, Powers SW, Scher MS, Hershey AD. Migraine headaches and sleep disturbances in children. Headache. 2003;43(4):362–368. doi: 10.1046/j.1526-4610.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- 14.Vendrame M, Kaleyias J, Valencia I, Legido A, Kothare SV. Polysomnographic findings in children with headaches. Pediatr Neurol. 2008;39(1):6–11. doi: 10.1016/j.pediatrneurol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Kato T, Rompre P, Montplaisir JY, Sessle BJ, Lavigne GJ. Sleep bruxism: an oromotor activity secondary to micro-arousal. J Dent Res. 2001;80(10):1940–1944. doi: 10.1177/00220345010800101501. [DOI] [PubMed] [Google Scholar]
- 16.Ohayon MM, Li KK, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119(1):53–61. doi: 10.1378/chest.119.1.53. [DOI] [PubMed] [Google Scholar]
- 17.Kampe T, Edman G, Bader G, Tagdae T, Karlsson S. Personality traits in a group of subjects with long-standing bruxing behaviour. J Oral Rehabil. 1997;24(8):588–593. doi: 10.1046/j.1365-2842.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- 18.Kara MI, Yanik S, Keskinruzgar A, et al. Oxidative imbalance and anxiety in patients with sleep bruxism. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5):604–609. doi: 10.1016/j.oooo.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 19.da Silva AM, Oakley DA, Hemmings KW, Newman HN, Watkins S. Psychosocial factors and tooth wear with a significant component of attrition. Eur J Prosthodont Restor Dent. 1997;5(2):51–55. [PubMed] [Google Scholar]
- 20.Manfredini D, Landi N, Fantoni F, Segu M, Bosco M. Anxiety symptoms in clinically diagnosed bruxers. J Oral Rehabil. 2005;32(8):584–588. doi: 10.1111/j.1365-2842.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- 21.Tavares LM, da Silva Parente Macedo LC, Duarte CM, de Goffredo Filho GS, de Souza Tesch R. Cross-sectional study of anxiety symptoms and self-report of awake and sleep bruxism in female TMD patients. Cranio. 2016;34(6):378–381. doi: 10.1080/08869634.2016.1163806. [DOI] [PubMed] [Google Scholar]
- 22.Hermesh H, Schapir L, Marom S, et al. Bruxism and oral parafunctional hyperactivity in social phobia outpatients. J Oral Rehabil. 2015;42(2):90–97. doi: 10.1111/joor.12235. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira MT, Bittencourt ST, Marcon K, Destro S, Pereira JR. Sleep bruxism and anxiety level in children. Braz Oral Res. 2015:29. doi: 10.1590/1807-3107BOR-2015.vol29.0024. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira-Bacci Ado V, Cardoso CL, Diaz-Serrano KV. Behavioral problems and emotional stress in children with bruxism. Braz Dent J. 2012;23(3):246–251. doi: 10.1590/s0103-64402012000300011. [DOI] [PubMed] [Google Scholar]
- 25.Restrepo CC, Vasquez LM, Alvarez M, Valencia I. Personality traits and temporomandibular disorders in a group of children with bruxing behaviour. J Oral Rehabil. 2008;35(8):585–593. doi: 10.1111/j.1365-2842.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 26.Türkoğlu S, Akca OF, Turkoglu G, Akca M. Psychiatric disorders and symptoms in children and adolescents with sleep bruxism. Sleep Breath. 2014;18(3):649–654. doi: 10.1007/s11325-013-0928-y. [DOI] [PubMed] [Google Scholar]
- 27.Lindqvist B. Bruxism in children. Odontol Revy. 1971;22(4):413–423. [PubMed] [Google Scholar]
- 28.De Luca Canto G, Singh V, Conti P, et al. Association between sleep bruxism and psychosocial factors in children and adolescents: a systematic review. Clin Pediatr (Phila) 2015;54(5):469–478. doi: 10.1177/0009922814555976. [DOI] [PubMed] [Google Scholar]
- 29.Silverman W, Albano A. The Anxiety Disorders Interview Schedule for Children-IV (Child and parent versions) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 30.Lyneham HJ, Abbott MJ, Rapee RM. Interrater reliability of the Anxiety Disorders Interview Schedule for DSM-IV: child and parent version. J Am Acad Child Adolesc Psychiatry. 2007;46(6):731–736. doi: 10.1097/chi.0b013e3180465a09. [DOI] [PubMed] [Google Scholar]
- 31.Silverman WK, Saavedra LM, Pina AA. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: child and parent versions. J Am Acad Child Adolesc Psychiatry. 2001;40(8):937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- 33.Iber C, Ancoli-Israel S, Chesson A, Quan S for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 34.Carra MC, Huynh N, Lavigne GJ. Diagnostic accuracy of sleep bruxism scoring in absence of audio-video recording: a pilot study. Sleep Breath. 2015;19(1):183–190. doi: 10.1007/s11325-014-0986-9. [DOI] [PubMed] [Google Scholar]
- 35.Carra MC, Huynh NT, El-Khatib H, Remise C, Lavigne GJ. Sleep bruxism, snoring, and headaches in adolescents: short-term effects of a mandibular advancement appliance. Sleep Med. 2013;14(7):656–661. doi: 10.1016/j.sleep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Alfano CA, Reynolds K, Scott N, Dahl RE, Mellman TA. Polysomnographic sleep patterns of non-depressed, non-medicated children with generalized anxiety disorder. J Affect Disord. 2013;147(1-3):379–384. doi: 10.1016/j.jad.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohmi H, Kato M, Meadows M. Relationship between type A behavior patterns and risk of temporomandibular disorder in Japanese undergraduate students. J Rural Med. 2016;11(2):77–80. doi: 10.2185/jrm.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahlberg J, Rantala M, Savolainen A, et al. Reported bruxism and stress experience. Community Dent Oral Epidemiol. 2002;30(6):405–408. doi: 10.1034/j.1600-0528.2002.00007.x. [DOI] [PubMed] [Google Scholar]
- 39.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(6):e26–e50. doi: 10.1016/j.tripleo.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Bendtsen L, Jensen R. Tension-type headache. Neurol Clin. 2009;27(2):525–535. doi: 10.1016/j.ncl.2008.11.010. [DOI] [PubMed] [Google Scholar]


