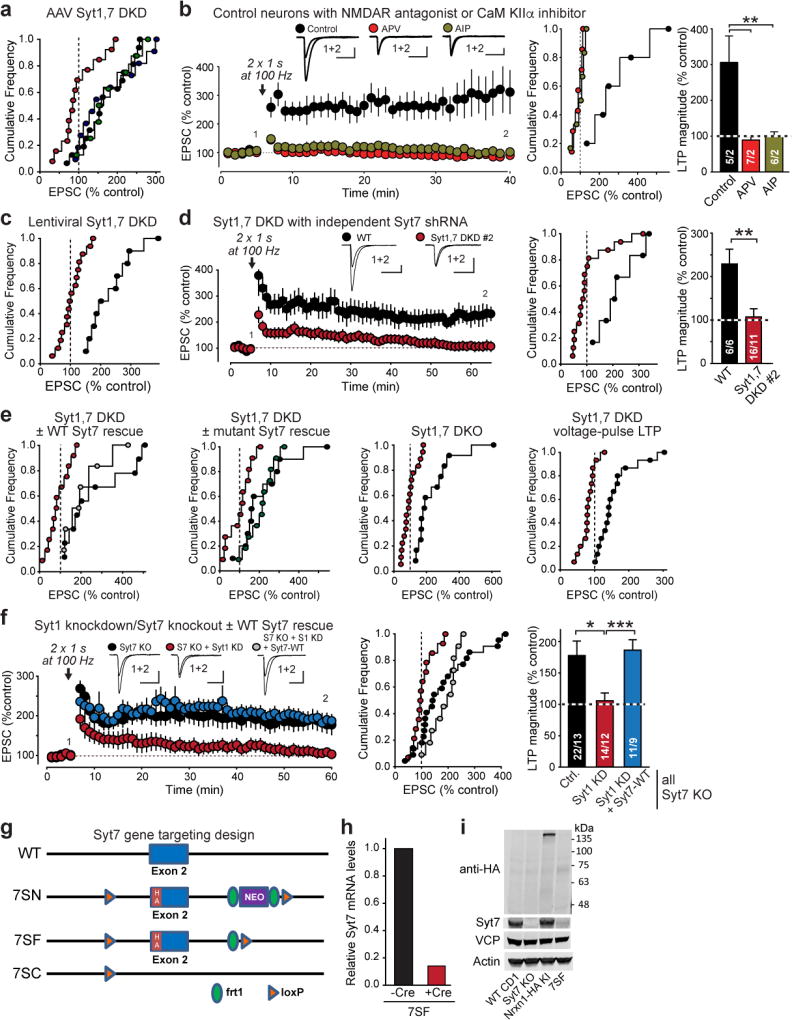
Extended Data Figure 3. Control experiments for LTP measurements, and characterization of a new Syt7 mutant mouse.
a, Cumulative frequency plots of LTP magnitude for acute slices from AAV-infected mice expressing control (black), Syt1 KD (green), Syt7 KD (blue) or Syt1,7 DKD virus (red). Data are from Fig. 1b.
b, APV (50 µM) in the extracellular solution or AIP (autocamide-2-related inhibitory peptide, 20 µM included in the pipette solution) impair LTP. Left, representative traces and LTP time course; center and right, cumulative frequency plots and summary graphs of LTP magnitude.
c, Cumulative frequency plots of LTP magnitude for acute slices from lentivirally infected mice expressing control (black) or Syt1,7 DKD virus (red). Data are from Fig. 1c.
d, Lentiviral in vivo Syt1,7 DKD with a second, independent shRNA against Syt7 impairs LTP.
e, Cumulative frequency plots of LTP magnitude for the experiments shown in Fig. 2a–d. In addl experiments, black and red denote the control and Syt1,7 double-deficiency condition; blue and green signify rescue with WT Syt7 or Syt7 C2A-domain mutant, respectively.
f, LTP is blocked by postsynaptic KD of Syt1 in constitutive Syt7 KO mice, but rescued by WT shRNA-resistant Syt7.
g, Schematic showing design of the new Syt7 mutant alleles. Using homologous recombination, exon 2 of the mouse Syt7 gene that encodes the transmembrane region was modified to introduce an HA-tag into the Syt7 protein N-terminal to the transmembrane region; in addition, a loxP site was introduced into the 5’ intron, and a neomycin resistance cassette (NEO) that was flanked by frt1 sites and was followed by a second loxP site was introduced in the 3’ intron. The initial mouse mutant was named 7SN; FLP recombination removed the NEO cassette to produce strain 7SF that per design should have expressed HA-tagged Syt7 but failed to do so (see panel f). 7SF at the same time was designed to also serve as a conditional KO (cKO) in which Cre recombination deletes exon 2 to produce mouse strain 7SC, which represents a true constitutive Syt7 KO since exon 2 is out-of-frame and encodes the vital transmembrane region.
h, Lentiviral Cre expression in cultured hippocampal 7SF neurons reduced Syt7 mRNA levels by ~90%, demonstrating the 7SF neurons express Syt7 mRNA and that the 7SF locus is a conditional KO.
i, Immunoblotting of brain homogenates from adult mice with the indicated genotypes using antibodies to the HA epitope, Syt7, VCP, or actin (the latter two as loading controls) shows that 7SF does not express Syt7 protein. Neither HA antibodies nor Syt7 antibodies detected Syt7 protein in 7SF mice designed to express HA-tagged but otherwise normal Syt7 (see panel d). Immunoblots of proteins from wild-type CD1 mice and for another strain of Syt7 KO mice were included as positive and negative controls for Syt7, respectively; immunoblots of Nrxn1-HA knockin mice (unpublished) were used as a positive control for the HA-epitope immunoblot. Molecular weight markers indicated on right. For gel source data, see Supplementary Figure 1.
Data are means ± SEM (numbers in bars = number of neurons/mice analyzed). Statistical significance was assessed in a, c with Kruskal-Wallis ANOVA followed by pairwise comparisons with the Mann-Whitney U test, and in b by the Mann-Whitney U test (*, p<0.05; **, p<0.01; ***, p<0.001). Calibration bars = 50 pA, 50 ms for a–c.