Abstract
We developed a pesticide residue burden score (PRBS) based on a food frequency questionnaire and surveillance data on food pesticide residues to characterize dietary exposure over the past year. In the present study, we evaluated the association of the PRBS with urinary concentrations of pesticide biomarkers. Fruit and vegetable (FV) intake was classified as having high (PRBS ≥ 4) or low (PRBS < 4) pesticide residues for 90 men from the EARTH study. Two urine samples per man were analyzed for seven biomarkers of organophosphate and pyrethroid insecticides, and the herbicide 2,4-dichlorophenoxyacetic acid. We used generalized estimating equations to analyze the association of the PRBS with urinary concentrations of pesticide biomarkers. Urinary concentrations of pesticide biomarkers were positively related to high pesticide FV intake but inversely related to low pesticide FV intake. The molar sum of urinary concentrations of pesticide biomarkers was 21% (95% confidence interval (CI): 2%, 44%) higher for each one serving/day increase in high pesticide FV intake, and 10% (95% CI: 1%, 18%) lower for each one serving/ day increase in low pesticide FV intake. Furthermore, intake of high pesticide FVs positively related to most individual urinary biomarkers. Our findings support the usefulness of the PRBS approach to characterize dietary exposure to select pesticides.
Keywords: biomonitoring, dietary exposure, pesticides
INTRODUCTION
Human exposure to pesticides is ubiquitous. More than 90% of the US population has detectable concentrations of pesticide biomarkers in their urine or blood.1 Although pesticide exposure occurs through a variety of routes, diet especially intake of fruits and vegetables is the major exposure pathway to these chemicals in the general population. According to the US Pesticide Monitoring Program, fruits and vegetables have a considerably higher percentage of detectable pesticide residues and higher percentage of samples with residue exceeding the tolerance level than any other foods.2 Others have shown that intake of vegetables, but not other food groups, was positively related to urinary concentrations of metabolites of pyrethroid insecticides,3 and that substituting conventionally grown produce with organic produce dramatically decreases the urinary concentrations of select pesticide metabolites.4–6
Urinary biomarkers are commonly used for assessment of contemporary, non-persistent pesticide exposure. However, short half-lives, the episodic nature of exposure,7–10 lack of specificity for exposure to the parent pesticides,11,12 and high analytic costs may complicate the interpretation of urinary biomarker data and limit their utility in large-scale studies. We previously developed a low-cost, questionnaire-based method — the dietary pesticide residue burden score (PRBS) — to estimate exposure to pesticide residues from foods in epidemiologic studies.13,14 This approach can make it possible to explore hypotheses regarding the potential health effects of these chemicals quickly and economically. Nonetheless, the usefulness of this approach and the ability to extend its use relies on the extent that the PRBS can adequately characterize individuals’ exposure when compared with traditional biomarkers of pesticide exposure.
We previously showed using data from the National Health and Nutrition Examination Study (NHANES) that the PRBS can reasonably rank individuals’ pesticide exposure through diet when compared against urinary concentrations of non-specific metabolites of organophosphate insecticides.15 However, pesticide exposure in that study was based on the concentrations of pesticides biomarkers in a single spot urine sample, which may result in exposure missclassification given the high within-person variability in urinary concentrations of the biomarkers.7–10 In addition, the relationship with urinary pyrethroid metabolites has not been evaluated, which is particularly important as the use of pyrethroids is gaining popularity because they have become an available alternative to organophosphate insecticides.16
The present study aimed to validate the PRBS in a well-established longitudinal cohort by using two urine samples per participant to characterize exposures to commonly used pesticides, including organophosphate and pyrethroid pesticides, two of the most commonly used classes of insecticides, and 2,4-dichlorophenoxyacetic acid, an herbicide currently in use for broadleaf weed control in agricultural and non-agricultural settings.17
MATERIALS AND METHODS
Study Population
The study population comprised men participating in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort study evaluating the relationship of environmental and nutritional factors with fertility among couples presenting to the Massachusetts General Hospital Fertility Center (Boston, MA, USA). In April 2007, a food frequency questionnaire (FFQ) was introduced into the study to assess diet. Of 164 men who had completed a FFQ and provided at least two urine samples between April 2007 and July 2015, we selected 90 men, whose urine samples were collected within 9 months before or after FFQ completion, to have their stored urine samples analyzed for urinary pesticide biomarkers. Of the 180 samples, three (from three men) had record errors, leaving 177 samples available for analysis.
Upon study entry, men underwent an anthropometric assessment and completed a nurse-administered questionnaire in which basic demographic data were collected. Participants also completed a detailed take-home questionnaire, which contained questions on various lifestyle factors including pesticide exposure history, organic fruit and vegetable consumption frequency, and physical activity. Specifically, men were asked if their homes had been treated with pesticides in the past 5 years, if their lawns had been treated with pesticides in the past year, if they had used pesticide products personally or on pets to repel or kill pests in the past year, and if anyone in their household had been treated for head lice in the past year. Men were considered to have a history of recent residential pesticide exposure if they replied “yes” to any of these questions. Participants were also asked how often they consumed one serving of organic fruits and vegetables per day during the past 3 months. Men were considered as organic fruit and vegetable consumers if they consumed organic FVs ≥ 3 servings per week of organic fruits and vegetables; men with a lower intake of organic fruits and vegetables were considered as conventional fruit and vegetable consumers. We calculated total physical activity (hrs/week) according to participants’ time spent in physical activities at enrollment using a validated questionnaire.18 The study was approved by the Human Subjects Committees of the Harvard T.H. Chan School of Public Health and the Massachusetts General Hospital, and the Centers for Disease Control and Prevention.19 Informed consent was obtained from all participants.
Dietary Assessment
Diet was assessed using a previously validated 131-item FFQ.20 Men were asked to report how often, on average, they had consumed specified amounts of each food, beverage, and supplement in the questionnaire over the past year. The serving sizes for fruits and vegetables were described specifically for each item in the FFQ using standard portion sizes (e.g., one apple, ½ avocado) or volumes (e.g., ½ cup of broccoli). In a validation study21 the de-attenuated correlation (i.e., corrected for random within-person variability) between two, one-week diet records and FFQ reports ranged from 0.27 for spinach to 0.95 for banana.
Pesticide Residue Assessment
We assessed pesticide residues in fruits and vegetables using data from US Department of Agriculture’s Pesticide Data Program, a national program started in 1991 that annually tests agricultural commodities in the USA for the presence of ~ 450 different pesticide residues.22 To best represent the pesticide residues in the food supply, the Pesticide Data Program collects samples from 10 or more participating States comprising 50% of the nation’s population. Before testing, the produce is either washed or peeled to mimic consumer practices, allowing for realistic estimates of exposure. To determine the average pesticide residue status of fruits and vegetables, we developed the PRBS using the Pesticide Data Program annual reports corresponding to the periods in which the diet history of the participants was captured by the FFQ.22 Briefly, we defined PRBS13 according to three contamination measures from the Pesticide Data Program: (1) the percentage of samples tested with any detectable pesticides; (2) the percentage of samples tested with pesticides exceeding tolerance levels; and (3) the percentage of samples with three or more individual detectable pesticides. We ranked the 36 FVs included in the FFQ according to each of the three contamination measures, divided them into tertiles for each of these three measures, and assigned each food a score of 0, 1, and 2 corresponding to the bottom, middle, and top tertile, respectively. The final PRBS for each food was the sum of tertile scores across the three PDP contamination measures (Supplementary Table 1). We classified foods with a PRBS ≥ 4 as high pesticide residue foods and those with a PRBS < 4 as low pesticide residue foods.13 To derive a PRBS specific to a class of pesticides, we used a similar algorithm (i.e., three contamination measures) but restricted Pesticide Data Program data to organophosphates and pyrethroids only for calculating organophosphate-PRBS and pyrethroid-PRBS, respectively. In sensitivity analyses, we also considered an alternate measure, PRBS-weighted fruit and vegetable intake, calculated as the product of each food’s PRBS score (on a scale of 0 to 6) and its intake frequency.
Urine Pesticide Biomarker Measurements
Men collected spot urine samples at the baseline and follow-up clinic visits in sterile polypropylene cups. Specific gravity was measured using a handheld refractometer (National Instrument Company, Baltimore, MD, USA). The urine was aliquoted and stored at − 80 °C. Samples were shipped on dry ice overnight to the CDC (Atlanta, GA, USA) where they were analyzed for seven pesticide biomarkers: three organophosphate metabolites: 3,5,6-trichloro-2-pyridinol (TCPY), a metabolite of chlorpyrifos and chlorpyrifos-methyl; 2-isopropyl-4-methyl-6-hydroxy-pyrimidine (IMPY), a metabolite of diazinon; and para-nitrophenol (PNP), a metabolite of parathion and methyl parathion; three metabolites of pyrethroids: 4-fluoro-3-phenoxybenzoic acid (4F-3-PBA), a metabolite of cyfluthrin; trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (trans-DCCA), a metabolite of permethrin, cypermethrin, and cyfluthrin; and 3-phenoxybenzoic acid (3-PBA), a non-specific metabolite of cyhalothrin, cypermethrin, deltamethrin, fenpropathrin, permethrin, and tralomethrin; and one chlorophenoxy herbicide, 2,4-dichlorophenoxyacetic acid (2,4-D) (Supplementary Table 2). Solid phase extraction and high-performance liquid chromatography-isotope dilution tandem mass spectrometry was used to quantify the concentrations of these biomarkers. Procedure details and quality control procedures are described elsewhere.23 Due to presence of interfering compounds, IMPY concentrations in 19 urine samples could not be quantified. The limit of detection (LOD) for each biomarker is shown in Table 2.
Table 2.
Distributions of urinary concentrations (µg/l) of pesticide biomarkers in 177 urine samples from 90 men from the Environment and Reproductive Health (EARTH) Study.
| Class | Na | Detection frequencyb |
Geometricc mean 1919(USDA)19 |
Percentile | LOD | NHANES GMd |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| min | 25th | 50th | 75th | 90th | 95th | max | |||||||
| TCPY | OP | 177 | 77% | 0.61 (0.07) | < LOD | 0.29 | 0.68 | 1.4 | 2.23 | 2.66 | 5.93 | 0.1 | 0.865 |
| SG-adjusted TCPY | 0.69 (0.07) | < LOD | 0.31 | 0.83 | 1.26 | 1.97 | 2.91 | 6.62 | 0.77e | ||||
| PNP | OP | 177 | 100% | 0.74 (0.06) | 0.18 | 0.41 | 0.74 | 1.24 | 1.73 | 2.09 | 12.09 | 0.1 | 0.52 |
| SG-adjusted PNP | 0.84 (0.05) | 0.21 | 0.58 | 0.79 | 1.09 | 1.67 | 2.51 | 9.54 | 0.47e | ||||
| IMPY | OP | 158 | 53% | 0.15 (0.01) | < LOD | < LOD | 0.12 | 0.27 | 0.39 | 0.53 | 2.75 | 0.1 | – |
| SG-adjusted IMPY | 0.17 (0.01) | < LOD | 0.11 | 0.18 | 0.25 | 0.37 | 0.43 | 0.9 | – | ||||
| 3-PBA | PYR | 177 | 88% | 0.50 (0.06) | < LOD | 0.24 | 0.39 | 0.92 | 2.54 | 4.32 | 13.77 | 0.1 | 0.42 |
| SG-adjusted 3-PBA | 0.57 (0.06) | < LOD | 0.26 | 0.45 | 1.03 | 2.42 | 3.26 | 12.81 | 0.38e | ||||
| 4F-3-PBA | PYR | 177 | 9% | < LOD | < LOD | < LOD | < LOD | < LOD | 0.1 | 0.13 | 0.27 | 0.1 | – |
| SG-adjusted 4F 3-PBA | < LOD | < LOD | < LOD | < LOD | 0.13 | 0.2 | 0.24 | 0.56 | – | ||||
| trans-DCCA | PYR | 177 | 17% | < LOD | < LOD | < LOD | < LOD | 0.86 | 2.01 | 4.55 | 17.63 | 0.6 | – |
| SG-adjusted t-DCCA | < LOD | < LOD | < LOD | 0.63 | 1.27 | 2.14 | 3.5 | 16.4 | – | ||||
| 2,4-D | H | 177 | 78% | 0.31 (0.02) | < LOD | 0.19 | 0.30 | 0.47 | 0.7 | 0.91 | 1.57 | 0.15 | 0.347 |
| SG-adjusted 2,4-D | 0.35 (0.02) | < LOD | 0.24 | 0.36 | 0.5 | 0.69 | 0.96 | 1.95 | 0.309e | ||||
Abbreviations: GM, geometric mean; H, herbicide; IMPY, 2-isopropyl-4-methyl-6-hydroxy-pyrimidine; < LOD, below limit of detection; max, maximum; min, minimum; N, number of urine samples; NHANES, National Health and Nutrition Examination Survey; OP, organophosphate pesticides; PNP, para-nitrophenol; PYR, pyrethroids; SG-adjusted, specific gravity adjusted; trans-DCCA, trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid; TCPY, 3,5,6-trichloro-2-pyridinol; 2,4-D, 2,4-dichlorophenoxyacetic acid; 3-PBA, 3-phenoxybenzoic acid; 4F-3-PBA, 4-fluoro-3-phenoxybenzoic acid.
Number of urine samples.
Percent of metabolite concentrations above the LOD of the urine samples.
All concentrations < LOD were assigned a value equal to the LOD divided by √2 to calculate the geometric means of urinary pesticide metabolite.
Geometric mean for US male population from NHANES 2009–2010.
Creatinine was used to correct for urine dilution in NHANES. Therefore these values (µg/creatinine) represent creatinine-adjusted concentrations.
Statistical Analysis
We adjusted biomarker concentrations for urine dilution using the formula Pc = P((1.015 − 1)/specific gravity − 1), where Pc is the specific gravity-adjusted pesticide biomarker concentration (µg/l), P is the measured pesticide biomarker concentration (µg/l), and 1.015 is the mean specific gravity concentration in the study population.24 Non-detectable pesticide biomarker concentrations were replaced with a value equal to the LOD divided by square root of 2 before specific gravity adjustment.25 To quantify variability in urinary pesticide biomarkers, we calculated the intraclass correlation coefficient (ICC)26 based on the estimates of within-and between-subject variance obtained from the repeated measures in mixed effect models. Due to low detection rates for 4F-3-PBA and trans-DCCA concentrations, these two biomarkers were not considered in the following analyses.
To estimate the total pesticide burden based on urinary concentrations of pesticide biomarkers, we calculated the molar sum of the biomarkers (in µmol/l) by dividing each biomarker concentration by its molecular weight and then summing all concentrations across biomarkers. The molar sum was also calculated separately for each class of pesticides. We also ranked the participants according to each urinary pesticide concentration, and summed the ranks across the urinary biomarkers for each participant. Of note, for the summary measures of pyrethroid insecticide metabolites, we only used the data from 3-PBA, which is a non-specific metabolite of a wide class of pyrethroids.27 Intake of fruits and vegetables (i.e., high pesticide fruit and vegetable intake, low pesticide fruit and vegetable intake, and PRBS-weighted fruit and vegetable intake) was modeled as continuous variables as well as in quartiles. We used linear regression with generalized estimating equations to evaluate the relation of fruit and vegetable intake (modeled as independent variables) with specific gravity-adjusted individual pesticide biomarkers as well as the overall molar sum (modeled as dependent variables), while accounting for within-person correlations in repeated samples of the same individual. Specific gravity-adjusted urinary pesticide biomarkers were log-transformed to meet normality assumptions of linear regression. Resulting coefficients were back transformed to improve interpretability. Models were adjusted for age (years), body mass index (BMI) (kg/m2), total physical activity (h/week), race (white or non white), smoking status (ever or never), education levels (some college or lower, or college graduate), organic fruit and vegetable consumption (< 3 times per week, or ≥ 3 times per week), years and season (spring, summer, fall, or winter) of urine sample collection, and recent residential pesticide exposure history (yes or no) with the goal of decreasing extraneous variation in urinary biomarker concentrations.28 Models for high pesticide residue fruit and vegetable intake were additionally adjusted for low pesticide fruit and vegetable intake, and vice versa, as intake of high pesticide fruits and vegetables and low pesticide fruits and vegetables may confound each other. Robust estimators of variance were used to compute 95% confidence intervals (CIs). Population marginal means were utilized to present population averages adjusted for the covariates29 at their average level for continuous covariates and reference level for categorical variables. Tests for linear trend were performed using median intake of fruits and vegetables in each quartile as a continuous variable. In addition, we calculated the de-attenuated Spearman correlation (i.e., observed correlation corrected for within-person variability) between high/low pesticide fruit and vegetable intake and molar sum of urinary pesticide biomarkers.30
We also conducted additional sensitivity analyses in which we excluded the urine samples provided more than 6 months before or after FFQ completion. In addition, effect modification by organic food consumption (< 3 times per week vs ≥ 3 times per week), and recent residential pesticide exposure history (yes vs no) was tested using cross-product terms in the multivariable model. Lastly, we evaluated the association between organic fruit and vegetable consumption frequency (in quartiles) and the molar sum of urinary pesticide biomarkers adjusting for total fruit and vegetable intake and the same set of covariates in the main model. Statistical analyses were performed with SAS v9.4 (SAS Institute, Cary, NC, USA). Two-sided P-values < 0.05 were considered significant.
RESULTS
Most of the 90 men were white (89%), nonsmokers (68%), overweight or obese (68%), and their median age was 36.1 years (Table 1). The median (25th, 75th percentile) intake of fruits and vegetables was 3.6 (2.6, 5.1) servings/day. Seventy-three men (81%) reported a history of residential pesticide exposure in the past year. Approximately one fourth of the participants reported consuming organic fruits and vegetables three times or more per week. Organic fruit and vegetable consumers had higher intakes of both high pesticide (mean: 2.1 vs 1.2 servings/day) and low pesticide (mean: 3.2 vs 2.5 servings/day) fruits and vegetables than conventional fruit and vegetable consumers. The molar sum of urinary pesticide biomarkers was the same among organic and conventional fruit and vegetable consumers (mean: 17 µmol/l).
Table 1.
Baseline Characteristics of the 90 men contributing 177 urine samples from the Environment and Reproductive Health (EARTH) Study.
| Characteristic of men | Median (25th, 75th) or N (%) |
|---|---|
| Number of men | 90 |
| Demographics | |
| Age, years | 36.1 (33.8, 40.4) |
| BMI, kg/m2 | 27.0 (23.7, 28.9) |
| Total physical activity, hours/week | 6.0 (2.9, 10.5) |
| Never smokers, n (%) | 61 (68) |
| Race, n (%) | |
| White | 80 (89) |
| Black/African Americans | 1 (1) |
| Asian | 6 (7) |
| Others | 3 (3) |
| College graduates or higher, n (%) | 72 (80) |
| Consumed organic FVs ≥ 3 times/week, n (%) | 24 (27) |
| Residential pesticide exposure, n (%) | 66 (73) |
| Diet | |
| High pesticide FV intake, servings/day | 1.2 (0.8, 1.8) |
| Low pesticide FV intake, servings/day | 2.5 (1.6, 3.2) |
| Total energy intake, kcal/day | 2045 (1592, 2470) |
| Characteristics of urine samples | |
| Number of urine samplesa | 177 |
| Year of urine sample collection | 2010 (2009, 2011) |
| Time from FFQ completion to urine sample collection, days | 78 (−14, 165) |
| Season of urine sample collection, n (%) | |
| Spring | 40 (23) |
| Summer | 39 (22) |
| Fall | 44 (25) |
| Winter | 54 (31) |
Abbreviations: BMI, body mass index; FFQ, food frequency questionnaire; N, number.
Of 90 men, 87 men had 2 urine samples analyzed and 3 men had 1 urine sample analyzed.
All pesticide biomarkers were detected in over 50% of samples, except for 4F-3-PBA and trans-DCCA which were detected only in 8.5% and 17% of the samples, respectively (Table 2). The SG-adjusted geometric mean urinary concentrations for the 177 samples from 90 men were 0.69 (TCPY), 0.84 (PNP), 0.57 (IMPY), 0.38 (3-PBA) and 0.35 (2,4-D) µg/l (Table 2). These urinary pesticide biomarkers had low reproducibility with ICCs ranging from 0.03 (TCPY) to 0.37 (3-PBA) (Supplementary Table 2).
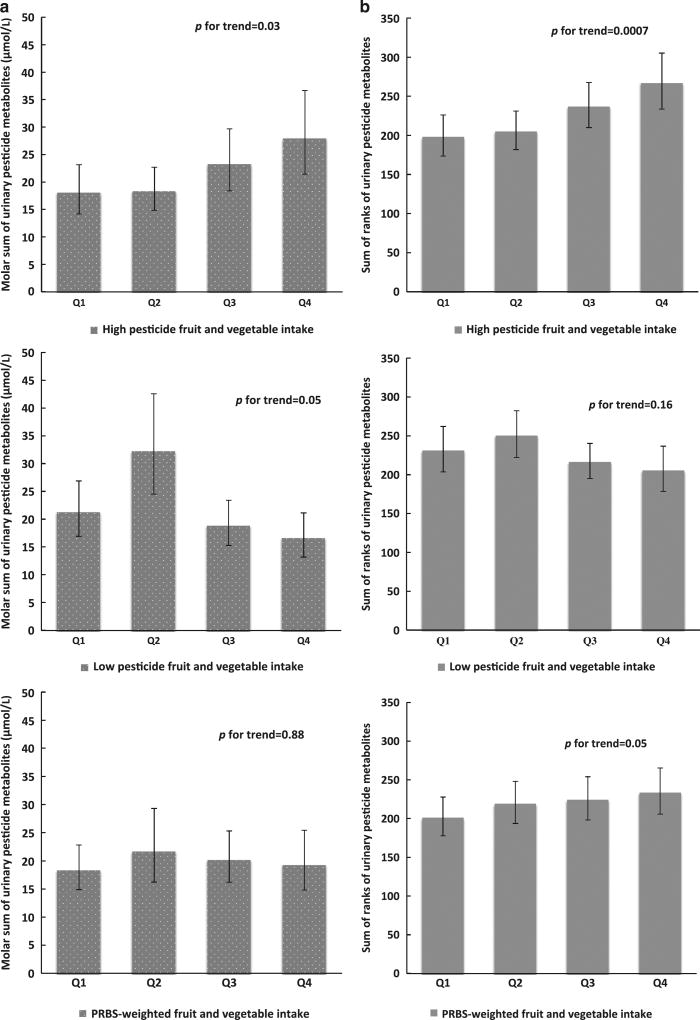
The PRBS for high residue fruits and vegetables was positively related to urinary pesticide biomarker concentrations (de-attenuated r = 0.55). In the unadjusted analysis, the molar sum of urinary pesticide biomarkers was 20% (95% CI: − 1%, 44%) higher for each one serving/day increase in high pesticide fruit and vegetable intake. Multivariable adjustment slightly strengthened the association, with the molar sum of urinary pesticide biomarkers increasing by 21% (95% CI: 2%, 44%) per one serving increase in high pesticide fruit and vegetable intake. Results were similar when consumption of high pesticide fruit and vegetable was modeled in quartiles of intake. Specifically, adjusted molar sum of urinary pesticide biomarkers were 18, 18, 23, and 28 µmol/l for men in increasing quartiles of high pesticide fruit and vegetable intake (p, trend = 0.03; Figure 1a). When each urinary pesticide was assessed individually, positive trends for high pesticide fruit and vegetable intake and urinary pesticide biomarkers were observed for most individual pesticides except for TCPY (Table 3). These associations were stronger when the analysis was restricted to urine samples collected within 6 months of FFQ completion (Supplementary Table 3). On the other hand, PRBS for low residue fruits and vegetables were negatively associated with urinary pesticide biomarkers concentrations (de-attenuated r = − 0.36). The unadjusted and adjusted molar sum of urinary pesticide biomarkers were 9% (95% CI: 1%, 19%) and 10% (95% CI: 1%, 18%) lower, respectively, for each one serving/day increase in low pesticide fruit and vegetable intake. The PRBS for low residue fruits and vegetables, when modeled as quartile variable, was also inversely related to the molar sum of urinary pesticide biomarkers (p, trend = 0.05; Figure 1a) but unrelated to any of the individual urinary biomarkers (Table 3). Furthermore, the PRBS-weighted fruit and vegetable intake was unrelated to urinary pesticide biomarkers (Figure 1a). These results were similar when the sum of ranks of pesticide biomarkers was used as comparison (Figure 1b) and when we excluded three men without a second sample available for analysis.
Figure 1.
Sum of SG-adjusted urinary pesticide biomarkers according to quartile of high pesticide residue, low pesticide residue, and PRBS-weighted fruit and vegetable intake among 88 men (158 samples) in the Environment and Reproductive Health (EARTH) Study. Data are presented as predicted mean (95% CI) in each quartile adjusted for age, race, BMI, total physical activity, smoking status, education, organic fruit, and vegetable consumption, years and season of urine sample collections, and residential pesticide use history. Summed pesticide biomarkers included TCPY, IMPY, PNP, 3-PBA, and 2,4-D. (a) Molar sum of the urinary pesticide biomarkers (µmol/l). (b) Sum of ranks of the urinary pesticide biomarkers.
Table 3.
Individual concentrations of SG-adjusted urinary pesticide biomarkers according to quartile of high/low pesticide residue and PRBS-weighted fruit and vegetable intake among 90 men in the Environment and Reproductive Health Study.
| Adjusteda mean (95% CIs) of individual pesticide metabolites (µg/l) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Organophosphate pesticide metabolites | Pyrethroid pesticide metabolites | Chlorophenoxy herbicide | |||
|
|
|
||||
| TCPY | IMPY | PNP | 3-PBA | 2,4-D | |
| Quartile (range) of high pesticide fruit and vegetable intake derived by PRBS | |||||
| Q1 (0.18, 0.79) | 1.19 (0.73, 1.93) | 0.16 (0.12, 0.22) | 0.60 (0.48, 0.77) | 0.57 (0.31, 1.06) | 0.34 (0.26, 0.43) |
| Q2 (0.81, 1.23) | 0.74 (0.48, 1.13) | 0.21 (0.15, 0.30) | 0.56 (0.44, 0.70) | 0.58 (0.34, 1.00) | 0.37 (0.26, 0.51) |
| Q3 (1.23, 1.80) | 0.93 (0.57, 1.51) | 0.25 (0.18, 0.35)b | 0.82 (0.61, 1.10) | 0.70 (0.41, 1.19) | 0.44 (0.34, 0.56) |
| Q4 (1.92, 4.34) | 1.56 (0.88, 2.76) | 0.24 (0.16, 0.35) | 0.85 (0.61, 1.20)b | 0.94 (0.56, 1.58) | 0.50 (0.36, 0.69)b |
| p, trendc | 0.16 | 0.24 | 0.04 | 0.17 | 0.03 |
| Quartile (range) of low pesticide fruit and vegetable intake derived by PRBS | |||||
| Q1 (0.36, 1.59) | 0.89 (0.53, 1.47) | 0.27 (0.19, 0.38) | 0.76 (0.59, 0.99) | 0.60 (0.36, 1.01) | 0.43 (0.34, 0.55) |
| Q2 (1.63, 2.52) | 1.34 (0.77, 2.33) | 0.25 (0.17, 0.35) | 0.86 (0.60, 1.23) | 1.09 (0.57, 2.06) | 0.43 (0.30, 0.63) |
| Q3 (2.56, 3.18) | 1.08 (0.67, 1.74) | 0.17 (0.12, 0.25) | 0.67 (0.54, 0.83) | 0.58 (0.36, 0.93) | 0.37 (0.30, 0.47) |
| Q4 (3.25, 9.31) | 0.99 (0.64, 1.52) | 0.18 (0.13, 0.24) | 0.54 (0.39, 0.74) | 0.58 (0.32, 1.06) | 0.38 (0.30, 0.49) |
| p, trendc | 0.91 | 0.11 | 0.13 | 0.59 | 0.48 |
| Quartile of PRBS-weighted fruit and vegetable intake | |||||
| Q1 | 1.02 (0.65, 1.60) | 0.20 (0.15, 0.27) | 0.60 (0.50, 0.72) | 0.55 (0.33, 0.92) | 0.34 (0.27, 0.41) |
| Q2 | 0.92 (0.58, 1.47) | 0.24 (0.17, 0.35) | 0.63 (0.48, 0.83) | 0.67 (0.36, 1.25) | 0.42 (0.30, 0.58) |
| Q3 | 0.83 (0.52, 1.35) | 0.21 (0.15, 0.29) | 0.76 (0.58, 1.00) | 0.57 (0.33, 0.98) | 0.41 (0.33, 0.52) |
| Q4 | 1.33 (0.80, 2.23) | 0.19 (0.13, 0.28) | 0.60 (0.45, 0.78) | 0.75 (0.47, 1.21) | 0.44 (0.33, 0.58) |
| p, trendc | 0.33 | 0.69 | 0.81 | 0.40 | 0.10 |
Abbreviations: IMPY, 2-isopropyl-4-methyl-6-hydroxy-pyrimidine; OP, organophosphate pesticides; PNP, para-nitrophenol; PRBS, pesticide residue burden score; PYR, pyrethroids; SG-adjusted, specific gravity adjusted; TCPY, 3,5,6-trichloro-2-pyridinol; 2,4-D, 2,4-dichlorophenoxyacetic acid; 3-PBA, 3-phenoxybenzoic acid.
Adjusting for age, race, BMI, total physical activity, smoking status, education, organic fruit and vegetable consumption, years and season of urine sample collections, and residential pesticide use history.
P-value < 0.05 compared with men in the lowest quartile of intake.
Estimated using median intake in each quartile as a continuous variable.
In analyses within class of pesticide, the PRBS based only on organophosphates for high pesticide fruits and vegetables were associated with higher urinary concentrations of organophosphate pesticides metabolites (Table 4). Specifically, men in the highest quartile of organophosphate-PRBS for high pesticide fruit and vegetable intake had 56% (95% CI: 8%, 125%) higher molar sum of organophosphate metabolites than men in the lowest quartile (p, trend = 0.02). Results were similar when the organophosphate-PRBS was compared against the sum of ranks of urinary organophosphate metabolites (Table 4). On the other hand, pyrethroid-PRBS for high pesticide fruit and vegetable intake were also positively related to the molar concentrations of 3-PBA and rank of the urinary 3-PBA concentration, albeit the association was weaker for molar concentration (Table 5).
Table 4.
Adjusteda mean of SG-adjusted urinary organophosphate pesticide metabolites according to organophosphate-PRBS derived high/low pesticide fruit and vegetable intake among 90 men in the Environment and Reproductive Health Study.
| Urinary organophosphate metabolites | ||
|---|---|---|
|
|
||
| Adjusteda mean (95% CI) in molar sumb (µmol/l) |
Adjusteda mean (95% CI) in sumb of ranks |
|
| Quartile (range) of high pesticide fruit and vegetable intake derived by organophosphate-PRBS | ||
| Q1 (0.08, 0.53) | 11 (9, 14) | 108 (92, 126) |
| Q2 (0.54, 0.89) | 10 (8, 12) | 107 (92, 125) |
| Q3 (0.90, 1.33) | 14 (11, 17) | 131 (115, 150)c |
| Q4 (1.35, 3.21) | 17 (12, 24)c | 142 (116, 174)c |
| p, trendd | 0.02 | 0.006 |
| Quartile (range) of low pesticide fruit and vegetable intake derived by organophosphate-PRBS | ||
| Q1 (0.46, 1.93) | 14 (10, 18) | 126 (105, 153) |
| Q2 (1.95, 2.74) | 17 (13, 22) | 129 (110, 152) |
| Q3 (2.76, 3.62) | 11 (9, 14) | 122 (105, 143) |
| Q4 (3.69, 10.4) | 10 (8, 12) | 107 (93, 124) |
| p, trendd | 0.03 | 0.08 |
Adjusting for age, race, BMI, total physical activity, smoking status, education, organic fruit and vegetable consumption frequency, years and season of urine sample collections, and residential pesticide use history.
Including 3,5,6-trichloro-2-pyridinol, 2-isopropyl-4-methyl-6-hydroxy-pyrimidine, and para-nitrophenol. Due to presence of interfering compounds in 19 samples for IMPY, only 158 samples (from 88 men) were available for molar sum organophosphate metabolite analysis.
P-value < 0.05 compared with men in the lowest quartile of intake.
Estimated using median intake in each quartile as a continuous variable.
Table 5.
Adjusted mean of SG-adjusted urinary pyrethroid pesticide metabolites according to pyrethroid-PRBS derived high/low pesticide fruit and vegetable intake among 90 men in the Environment and Reproductive Health Study.
| Urinary 3-phenoxybenzoic acid (3-PBA) | ||
|---|---|---|
|
|
||
| Adjusted mean (95% CI) in molar concentrationa (µmol/l) |
Adjusted mean (95% CI) in ranka |
|
| Quartile (range) of high pesticide fruit and vegetable intake derived by pyrethroid-PRBS | ||
| Q1 (0.16, 0.78) | 2.3 (1.2, 4.1) | 25 (15, 42) |
| Q2 (0.83, 1.37) | 2.8 (1.6, 4.9) | 34 (23, 52) |
| Q3 (1.39, 2.00) | 2.9 (1.7, 5.1) | 43 (29, 66)b |
| Q4 (2.06, 5.78) | 4.0 (2.4, 6.5) | 61 (43, 87)b |
| p, trendc | 0.15 | 0.01 |
| Quartile (range) of low pesticide fruit and vegetable intake derived by pyrethroid-PRBS | ||
| Q1 (0.32, 1.64) | 2.9 (1.7, 5.1) | 45 (30, 69) |
| Q2 (1.64, 2.30) | 3.4 (2.0, 5.8) | 45 (31, 64) |
| Q3 (2.31, 3.13) | 3.1 (1.9, 5.0) | 38 (26, 54) |
| Q4 (3.21, 7.87) | 1.9 (1.4, 4.4) | 30 (19, 49) |
| p, trendc | 0.48 | 0.13 |
Abbreviations: PRBS, pesticide residue burden score; SG-adjusted, specific gravity adjusted. Adjusting for age, race, BMI, total physical activity, smoking status, education, organic fruit and vegetable consumption, years and season of urine sample collections, and residential pesticide use history.
Uses 3-phenoxybenzoic acid as a biomarker of exposure to pyrethroids.
P-value < 0.05 compared with men in the lowest quartile of intake.
Estimated using median intake in each quartile as a continuous variable.
There was no statistical evidence of heterogeneity in the association between high pesticide fruit and vegetable intake and molar sum of urinary pesticide biomarkers according to organic fruit and vegetable consumption frequency, or recent residential pesticide exposure history (P, interaction >0.10 in all cases).
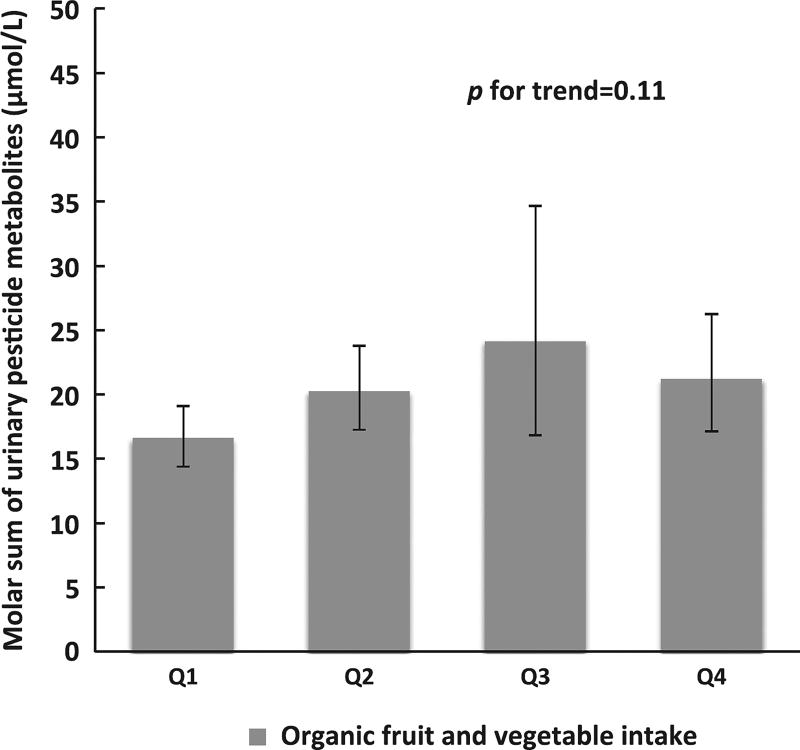
Lastly, we evaluated the association between frequency of organic fruit and vegetable consumption and molar sum of urinary pesticide biomarkers. The de-attenuated correlation coefficient was 0.36 between organic fruit and vegetable intake and molar sum of urinary pesticide biomarkers. There were no significant differences in urinary concentrations of pesticide biomarkers across quartile of organic fruit and vegetable consumption (Figure 2).
Figure 2.
Molar sum of SG-adjusted urinary pesticide biomarkers according to quartile of organic fruit and vegetable intake among 88 men (158 samples) in the Environment and Reproductive Health (EARTH) Study. Data are presented as predicted mean (95% CI) in each quartile adjusted for age, race, BMI, total physical activity, smoking status, education, total fruit and vegetable intake, years and season of urine sample collections, and residential pesticide use history. Summed pesticide biomarkers included TCPY, IMPY, PNP, 3-PBA, and 2,4-D. FV, fruit and vegetable.
DISCUSSION
We compared the PRBS against urinary pesticide biomarkers to evaluate the usefulness of a food frequency questionnaire method to estimate dietary intake of pesticides in epidemiologic studies. We found that intake of high pesticide fruit and vegetable was positively associated with urinary concentrations of pesticide biomarkers suggesting that this low-cost questionnaire-based method could be used as a tool to evaluate exposure in studies on health effects of pesticides before making the significant financial investment entailed in collecting and generating exposure biomonitoring data.
As objective measures reflecting aggregate exposure and internal dose, urinary biomarkers have been widely used to assess contemporary pesticide exposure in many studies.5,31–33 These biomarkers of exposure, however, are well known for having short half-lives, being sensitive to the episodic nature of exposure (reflected as low ICCs in the present study),7,8 relatively poor time integration and high analytic costs, limiting their use for long-term exposure assessment in epidemiologic studies when repeated measurements over years can not been obtained. On the other hand, the PRBS leverages the features of FFQ data, which reflect a longer period of dietary intake (i.e., a year) and is not as costly. Therefore, the PRBS approach can be useful in studies where the goal is to assess the effect of dietary pesticide exposure on chronic diseases, especially suitable for in cohorts with repeated FFQ measurements across years. In fact, the underlying principle of coupling a dietary questionnaire and national surveillance data, in the form of nutrient composition tables, has been widely implemented in nutritional epidemiology and used as a biologically meaningful measure of intake.28 Foods are vehicles for nutrients as well as non-nutritive constituent chemicals including pesticide residues. The present study shows that we may extend the coupling method to screen hypotheses regarding the potential health effects of pesticide residues as well.
The study findings complement the previous research from our group15 showing that PRBS had value as a surrogate for dietary organophosphate pesticide exposure. Hu et al. found that there was a dose response relationship between dietary pesticide exposure estimated by PRBS and urinary dialkylphosphate metabolites (non-specific organophosphate biomarkers) in 1918 adult participants from the 2003–2004 US National Health and Nutrition Examination Survey.15 In the present study conducted in a well-characterized longitudinal cohort, we further targeted three commonly used organophosphate pesticides, including chlorpyrifos, parathion, and diazinon, using two urine samples from each participant. Notably, these pesticides have been banned for indoor residential use since early 2000,34 suggesting that at present the major source of these chemicals is likely from diet, assuming that these pesticides were all used legally. In partial agreement with this hypothesis, we found positive trends of high pesticide fruit and vegetable intake with urinary concentrations of IMPY and PNP, and, to a lesser extent, TCPY. The weaker association with TCPY could be related to its high within-person variability (reflected in a low ICC), or to exposure to chlorpyrifos in public spaces such as golf-courses, turf, green houses, and wood treatment, that were not affected by the residential use ban;35 such uses could make this metabolite less specific to exposure via diet than the other two organophosphate metabolites studied.
In addition to evaluating specific metabolites of organophosphate pesticides, we added to the previous study15 by evaluating the association of pyrethroid-PRBS derived high pesticide fruit and vegetable intake with urinary concentrations of pyrethroid metabolites. There was a suggestive positive trend between high pesticide fruit and vegetable intake and molar sum of pyrethroid biomarkers. Interestingly, the distinguishability of PRBS was stronger for organophosphate than pyrethroid pesticides. This finding was not surprising as pyrethroids as a replacement for organophosphates in residential pest control,16 which may in turn, reduce the predictability of the urinary biomarkers for dietary exposure. In addition, the shorter half-life of pyrethroid pesticides (~5.7 h for 3-PBA) relative to certain organophosphate pesticides (~27 h for TCPY) may also partly explain the weaker association of the PRBS with pyrethroid relative to that with organophosphate pesticides.36,37 We found a significantly positive association between high pesticide fruit and vegetable intake and 2,4-D urinary concentrations. These findings are consistent with those of an organic diet intervention study among young children5 in which the investigators observed lower, albeit not significantly, 3-PBA urinary concentrations (P = 0.16), and significantly lower 2,4-D concentrations (P < 0.01) during the organic diet phase compared with the conventional phase. Lastly, and unexpectedly, we found that organic produce consumers and conventional produce consumers had similar pesticide biomarker urinary concentrations. One likely explanation was that organic produce consumers had higher fruit and vegetable intake and not all the consumed produce was organic. Alternatively, misclassification of organic fruit and vegetables intake cannot be ruled out as an online survey showed that among representative sample of 1005 U.S adult consumers, half of consumers think “natural” labeling means no pesticide,38 suggesting that assessing only the overall intake frequency of organic fruits and vegetables may be insufficient to characterize exposure to pesticides through diet. Taken together, our findings show that PRBS may serve a useful tool for assessment of long-term dietary exposure to selected pesticide residues, namely organophosphate insecticides, pyrethroid insecticides, and the herbicide 2,4-D.
A similar method was developed by Curl et al. to assess dietary organophosphate pesticide exposure in the Multi-Ethnic Study of Altherosclerosis (MESA).39 Briefly, Curl et al. estimated the organophosphate pesticide exposure in units of nanomoles per day for each individual by summing the product of average daily intake of each fruit and vegetable, concentration of organophosphate pesticides in each fruit and vegetable, and molecular weight of each organophosphate pesticide. Consistent with our findings, Curl et al. found that increasing tertiles of estimated exposure to dietary organophosphate pesticide were associated with higher urinary concentrations of dialkylphosphates. In comparison to the MESA score, our PRBS first identified produce with high vs low pesticide residue contamination, and then summed the intake of the fruits and vegetables with high and low pesticide residue, respectively. In spite of using different algorithms, both approaches correlate well with urinary pesticide exposure biomarkers. Nonetheless, it is worth highlighting that de-linking the potentially deleterious effect of pesticide residues from the beneficial components in fruits and vegetables remains a significant challenge in studies evaluating pesticide residue intake and associated health risks. While Curl et al.’s approach provided a quantitative measure of organophosphate pesticide exposure, our PRBS method created a “control” group—low pesticide fruit and vegetable intake, allowing us to compare the effect of high vs low pesticide residue on outcomes of interest in parallel while simultaneously accounting for overall intake of fruits and vegetables. In fact, a suggestive inverse association between low pesticide fruit and vegetable intake and urinary pesticide exposure as well as beneficial effect of low pesticide fruit and vegetable intake on semen quality shown in a previous study13 suggest that separating fruits and vegetables into high vs low pesticide residue content may be a viable approach to disentangle the health effects of fruits and vegetables from those of pesticide contamination of these foods.
Although the PRBS overcomes some of the shortcomings of urinary biomarkers including relatively high cost, non-specificity to parent compounds, high variability, and lack of time integration, the method is not without limitations. First, pesticide exposure may occur through other routes including inhalation and dermal contact, but the PRBS captures exposure only through dietary ingestion. Nonetheless, pharmacodynamic studies suggest that dermal and inhalation exposure to organophosphate and pyrethroid pesticides in the general population is likely to be relatively low due to poor dermal absorption (~1% excreted in urine37,40,41) and reduced volatility.42 Second, our estimates of pesticide residues in foods were based on surveillance data rather than actual pesticide residues in the food consumed by the participants. Nevertheless, the Pesticide Data Program includes selection at random of the food samples to be tested from supermarkets across the nation, and monthly sampling of foods over each 2-year cycle to allow measurement of seasonal and year-to-year variation in pesticide residue concentrations. Therefore, this design helps ensure that pesticide contamination values assigned to specific foods are reasonable estimates of the actual exposure concentration of any one person consuming foods sold in the United States. Third, PRBS does not take potency of toxicity of individual chemicals into account. Of note, because the aim of our paper was to investigate the relationship between the PRBS method and urinary biomarkers, we did not incorporate toxicity potency factors of individual pesticides into the score metric. However, future research on health effects of pesticide exposure should consider incorporating toxicity potency factors for each chemical into the PRBS method. Fourth, while we chose urinary biomarkers as a comparison measure for the PRBS method, it is important to emphasize that urinary biomarkers are not a gold standard for chornic exposure and in fact there is no “gold standard” to assess long-term exposure to contemporary, nonpersistent pesticides. Furthermore, comparison of the PRBS to urinary biomarkers was not expected to demonstrate strong correlation for several reasons including: (1) differences in time integration of exposure between FFQ and urinary biomarkers (i.e., FFQ captures dietary exposure in the past year while urinary biomarkers reflect exposure over past few hours or days; mismatch of time at FFQ and urinary sample assessment), (2) non-specificity of PRBS (i.e., PRBS captured overall pesticide exposure instead of targeting a certain pesticide biomarker), (3) non-dietary sources of exposure (i.e., PRBS captured dietary pesticide exposure while urinary biomarkers capture both dietary and non-dietary sources of exposure), (4) measurement error in the FFQ, and (5) high within-person variability in urinary pesticide biomarker concentrations. These could, individually and collectively, attenuate the observed association of dietary pesticide residue intake measured by PRBS and the urinary pesticide biomarker concentrations. Nonetheless, we used two urine samples to account for within-person variability in the concentrations of these chemicals, collected detailed information including residential exposure history, organic produce consumption frequency and years as well as season of urine sample collection, which allowed to remove extraneous variation attributed to these factors. Finally, the findings may not be generalizable to the US population because the participants were recruited through a fertility clinic, who have a higher social economic status and higher intake of fruits and vegetables (median: 3.6 servings/day) compared with the median intake in the U.S. population (median: 2.0 servings/day).43 Nevertheless, these results are consistent with our previous report comparing the PRBS to non-specific urinary pesticide biomarkers in NHANES,15 arguing against problems with generalizability.
In conclusion, the PRBS scoring system is a useful tool for dietary pesticide assessment in epidemiological studies aimed at evaluating hypotheses regarding the health effects of long-term exposure to pesticides through diet.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Mark Davis and William Roman (CDC, Atlanta, GA) for their technical assistance with pesticide biomarkers measurements. We also acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research staff Jennifer Ford, Myra Keller, and Ramace Dadd, physicians and staff at Massachusetts General Hospital fertility center. A special thank you to all of the study participants. Yu-Han Chiu gratefully acknowledges the doctoral scholarship from the Irene M. and Fredrick J. Stare Nutrition Education Fund. This research was funded by NIH grants R01ES022955, R01ES009718 and P01ES000002 from NIEHS and P30DK046200 from NIDDK; Harvard National Institute of Environmental Health Sciences (NIEHS) Center for Environmental Health Pilot Project Program. Qi Sun was supported by NIH grants ES021372 and ES022981. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
References
- 1.Atlanta, GA: USA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2015. Centers for Disease Control and Prevention Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2015. Available at http://www.cdc.gov/exposurereport. [Google Scholar]
- 2.US Food and Drug Administration Pesticide monitoring program fiscal year 2012 pesticide report 2012 [Google Scholar]
- 3.Fortes C, Mastroeni S, Pilla MA, Antonelli G, Lunghini L, Aprea C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food Chem Toxicol. 2013;52:91–96. doi: 10.1016/j.fct.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic diets significantly lower children's dietary exposure to organophosphorus pesticides. Environ Health Perspect. 2006;114:260–263. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradman A, Quiros-Alcala L, Castorina R, Schall RA, Camacho J, Holland NT, et al. Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ Health Perspect. 2015;123:1086–1093. doi: 10.1289/ehp.1408660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oates L, Cohen M, Braun L, Schembri A, Taskova R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res. 2014;132:105–111. doi: 10.1016/j.envres.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Wielgomas B. Variability of urinary excretion of pyrethroid metabolites in seven persons over seven consecutive days--implications for observational studies. Toxicol Lett. 2013;221:15–22. doi: 10.1016/j.toxlet.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Bradman A, Kogut K, Eisen EA, Jewell NP, Quiros-Alcala L, Castorina R, et al. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ Health Perspect. 2013;121:118–124. doi: 10.1289/ehp.1104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudakin DL, Stone DL. Dialkyl phosphates as biomarkers of organophosphates: the current divide between epidemiology and clinical toxicology. Clin Toxicol. 2011;49:771–781. doi: 10.3109/15563650.2011.624101. [DOI] [PubMed] [Google Scholar]
- 10.Spaan S, Pronk A, Koch HM, Jusko TA, Jaddoe VW, Shaw PA, et al. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. J Expo Sci Environ Epidemiol. 2015;25:286–294. doi: 10.1038/jes.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- 12.Krieger RI, Dinoff TM. Malathion deposition, metabolite clearance, and cholinesterase status of date dusters and harvesters in California. Arch Environ Contam Toxicol. 2000;38:546–553. doi: 10.1007/s002449910071. [DOI] [PubMed] [Google Scholar]
- 13.Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, et al. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod. 2015;30:1342–1351. doi: 10.1093/humrep/dev064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu YH, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Levine H, et al. Intake of fruits and vegetables with low-to-moderate pesticide residues is positively associated with semen-quality parameters among young healthy men. J Nutr. 2016;146:1084–1092. doi: 10.3945/jn.115.226563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y, Chiu Y-H, Hauser R, Chavarro J, Sun Q. Overall and class-specific scores of pesticide residues from fruits and vegetables as a tool to rank intake of pesticide residues in United States: a validation study. Environ Int. 2016;92:294–300. doi: 10.1016/j.envint.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ATSDR Toxicological Profile for Pyrethrins and Pyrethroids. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2003. [PubMed] [Google Scholar]
- 17.Environmental Protection Agency 2,4-D. [accessed 8 June 2016];2016 Available at https://www.epa.gov/ingredients-used-pesticide-products/24-d.
- 18.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 19.CDC Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; Washington, DC: 2009. [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 21.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 22.USDA, Agricultural Marketing Service; 2006–2015. USDA Pesticide Data Program (PDP), annual sumary. URL http://www.ams.usda.gov/AMSv1.0/PDP. [Google Scholar]
- 23.Davis MD, Wade EL, Restrepo PR, Roman-Esteva W, Bravo R, Kuklenyik P, et al. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J Chromatogr B. 2013;929:18–26. doi: 10.1016/j.jchromb.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120:1538–1543. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 26.Pastore LM, Hertz-Picciotto I, Beaumont JJ. Risk of stillbirth from occupational and residential exposures. Occup Environ Med. 1997;54:511–518. doi: 10.1136/oem.54.7.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starr J, Graham S, Stout D, 2nd, Andrews K, Nishioka M. Pyrethroid pesticides and their metabolites in vacuum cleaner dust collected from homes and day-care centers. Environ Res. 2008;108:271–279. doi: 10.1016/j.envres.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Willett W. Nutritional epidemiology: issues and challenges. Int J Epidemiol. 1987;16:312–317. doi: 10.1093/ije/16.2.312. [DOI] [PubMed] [Google Scholar]
- 29.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34:216–221. [Google Scholar]
- 30.Rosner B, Glynn RJ. Interval estimation for rank correlation coefficients based on the probit transformation with extension to measurement error correction of correlated ranked data. Stat Med. 2007;26:633–646. doi: 10.1002/sim.2547. [DOI] [PubMed] [Google Scholar]
- 31.Harley KG, Engel SM, Vedar MG, Eskenazi B, Whyatt RM, Lanphear BP, et al. Prenatal exposure to organophosphorous pesticides and fetal growth: pooled results from four longitudinal birth cohort studies. Environ Health Perspect. 2016;124:1084–1092. doi: 10.1289/ehp.1409362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu C, Barr DB, Pearson MA, Walker LA, Bravo R. The attribution of urban and suburban children's exposure to synthetic pyrethroid insecticides: a longitudinal assessment. J Expo Sci Environ Epidemiol. 2009;19:69–78. doi: 10.1038/jes.2008.49. [DOI] [PubMed] [Google Scholar]
- 33.Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA, USA: 2009. [Google Scholar]
- 35.Environmental Protection Agency. Proposal to Revoke Chlorpyrifos Food Residue Tolerances. [Accessed 8 July 2016];2016 Available at https://www.epa.gov/ingredients-used-pesticide-products/proposal-revoke-chlorpyrifos-food-residue-tolerances.
- 36.Ratelle M, Cote J, Bouchard M. Toxicokinetics of permethrin biomarkers of exposure in orally exposed volunteers. Toxicol Lett. 2015;232:369–375. doi: 10.1016/j.toxlet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Natural Marketing Institue Consumer insights on organic and natural. Organic& Natural Health Association; 2015. [Google Scholar]
- 39.Curl CL, Beresford SA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, et al. Estimating pesticide exposure from dietary intake and organic food choices: the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2015;123:475–483. doi: 10.1289/ehp.1408197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garfitt SJ, Jones K, Mason HJ, Cocker J. Exposure to the organophosphate diazinon: data from a human volunteer study with oral and dermal doses. Toxicol Lett. 2002;134:105–113. doi: 10.1016/s0378-4274(02)00178-9. [DOI] [PubMed] [Google Scholar]
- 41.Woollen BH, Marsh JR, Laird WJ, Lesser JE. The metabolism of cypermethrin in man: differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica. 1992;22:983–991. doi: 10.3109/00498259209049904. [DOI] [PubMed] [Google Scholar]
- 42.Poet TS, Timchalk C, Hotchkiss JA, Bartels MJ. Chlorpyrifos PBPK/PD model for multiple routes of exposure. Xenobiotica. 2014;44:868–881. doi: 10.3109/00498254.2014.918295. [DOI] [PubMed] [Google Scholar]
- 43.Moore LV, Dodd KW, Thompson FE, Grimm KA, Kim SA, Scanlon KS. Using behavioral risk factor surveillance system data to estimate the percentage of the population meeting US department of agriculture food patterns fruit and vegetable intake recommendations. Am J Epidemiol. 2015;181:979–988. doi: 10.1093/aje/kwu461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.