Figure 3. Endogenous proteins can bridge interactions between expressed proteins.
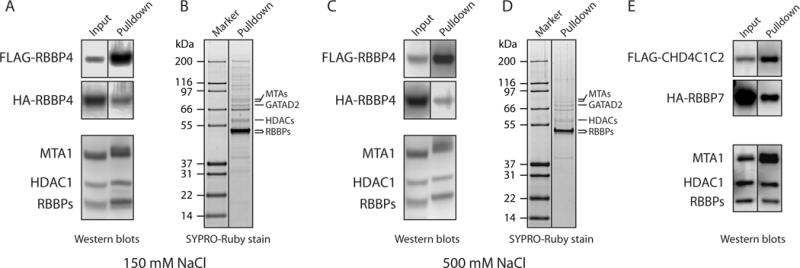
(a) Western blots showing investigation of a possible interaction between FLAG-RBBP4 and HA-RBBP4. The two proteins were co-expressed in HEK293 cells and purified on anti-FLAG beads with 150 mM NaCl washes. The blots were developed with anti-FLAG (top panel), anti-HA (middle panel) or a mixture of anti-MTA1, anti-HDAC1 and anti-RBBP4/7 (lower panel). Proteins detected in each band are indicated. In the middle panel, a band for HA-RBBP4 in the pulled down fraction suggests a direct RBBP4-RBBP4 interaction. However, endogenous NuRD components are detected in the lower blot that was run with a sample from the same pulldown. (b) SYPRO-Ruby stained gel of the purified protein fraction used in (a), showing that a number of endogenous proteins co-purify with FLAG-RBBP4. (c, d) Same as in (a, b), but anti-FLAG beads were washed with 500 mM NaCl during purification. Even under these higher stringency conditions, endogenous NuRD components were observed. (e) CoIP assessing a possible interaction between CHD4C1C2 (residues 1230–1912) and RBBP7. FLAG-CHD4C1C2 and HA-RBBP7 were co-expressed in HEK293 cells and FLAG-CHD4C1C2 was purified with anti-FLAG beads. Detection with anti-FLAG or anti-HA antibodies (top two panels) suggests a direct CHD4-RBBP7 interaction. However, endogenous NuRD components, which could be bridging the interaction, were also detected in the lower blot, which was run with a sample from the same pulldown and developed as in the lower panel in (a).
