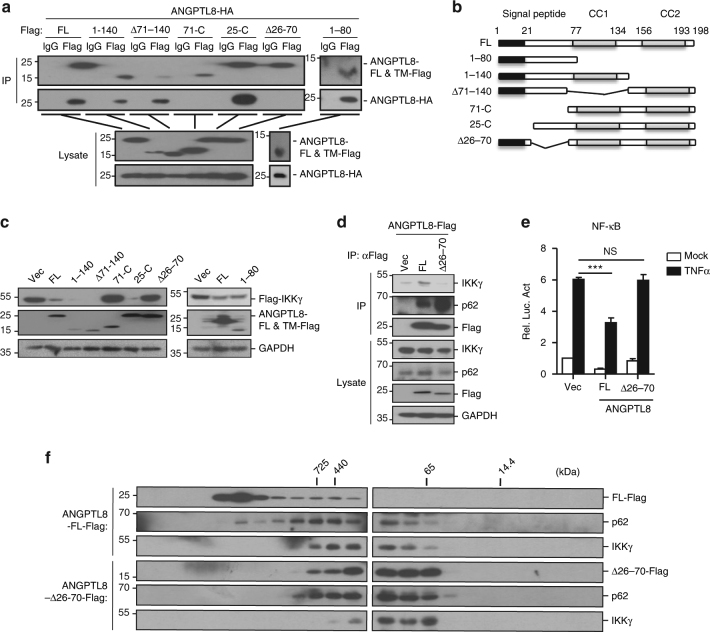
Fig. 9.
ANGPTL8 self-aggregation is essential for the degradation of IKKγ. a Domain mapping of the ANGPTL8 self-aggregation in HEK293T cells. b A schematic presentation of human ANGPTL8 and its truncation mutants. c The effects of ANGPTL8 and its truncation mutants on the degradation of IKKγ in HEK293T cells. d The ANGPTL8-Δ26–70 shows a diminished interaction with IKKγ. Three dishes of HEK293T cells (2 × 106 cells per dish) were transfected with indicated plasmids, 24 h later, cells were lysed and subjected to co-IP and followed immunoblots by indicated antibodies. e The effects of the full-length or −Δ26−70 truncation mutant of ANGPTL8 on the TNFα-induced NF-κB activation (n = 3). f Analysis of protein complex containing ANGPTL8, p62 and IKKγ by size-exclusion chromatography. HEK293T cells (2 × 106) were transfected with indicated plasmids for 24 h later before cells were lysed and subjected to size-exclusion chromatography. Data are shown as the mean ± SEM in e, unpaired two-tailed Student’s test was used for statistics (e). ***p < 0.0001, NS > 0.05. Data are representative of three independent experiments. FL full length, TM truncation mutants