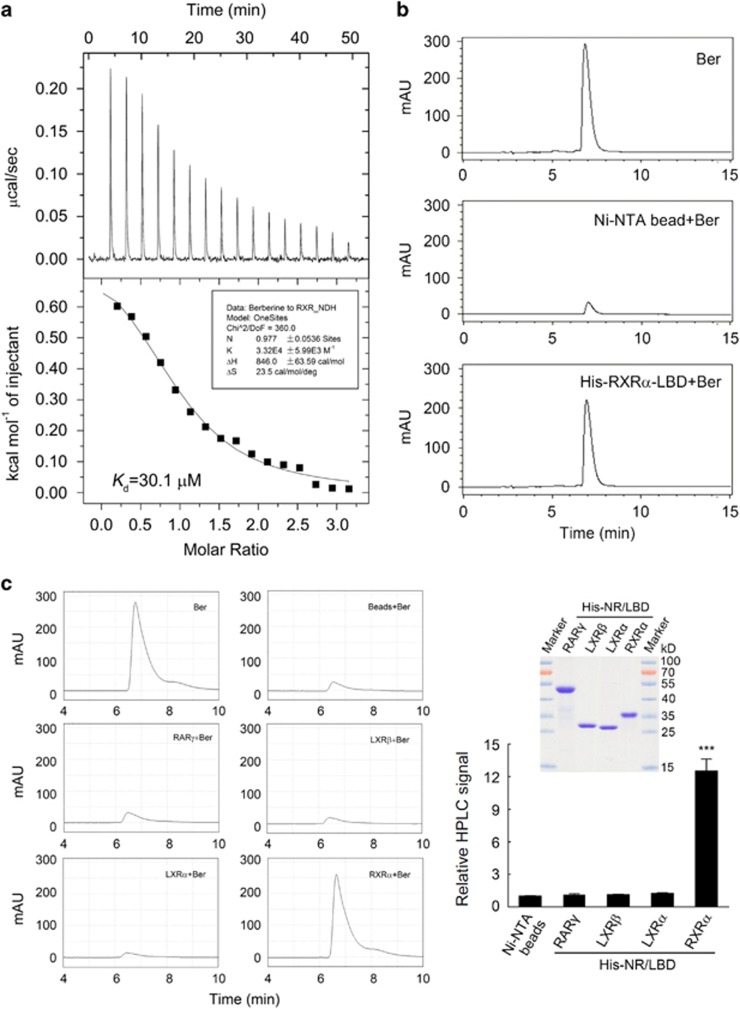
Figure 2.
Berberine directly bound to RXRα-LBD. (a, b) Analysis of berberine binding to RXRα-LBD by isothermal titration calorimetry (ITC) (a) and HPLC (b). (a) Purified RXRα-LBD protein was dialyzed against phosphate-buffered saline (PBS) buffer containing 4% DMSO and diluted to 0.1 mm with the same buffer. Berberine was dissolved to 1 mm in the same buffer, then the binding affinity and the number of binding sites of berberine to RXRα-LBD was measured by ITC. (b) Ni-NTA beads bound RXRα-LBD proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine at 4 °C, then the beads were collected after centrifugation and washed with PBS. The beads were dissolved in chloroform–methanol 1:1(v/v) and subjected to HPLC analysis as described in Materials and Methods. The same amount of berberine as mentioned above was used for identification and quantitative calculations. (c) HPLC analysis of berberine binding to the LBD of several nuclear receptors (NRs) including RARγ, LXRα and LXRβ. Ni-NTA beads bound NR-LBD proteins or Ni-NTA beads alone were incubated overnight with equal mole of berberine at 4 °C, then the beads were washed with PBS, dissolved in chloroform–methanol 1:1(v/v) and subjected to HPLC analysis. The HPLC signal indicating the relative binding affinity in all groups were normalized to the Ni-NTA beads group. Inserted right panel showed the expression levels of these NRs-LBD proteins, detected by SDS–PAGE with coomassie blue staining. All data were shown as the mean±s.e.m. of three independent experiments. ***P<0.001 vs Ni-NTA beads control.