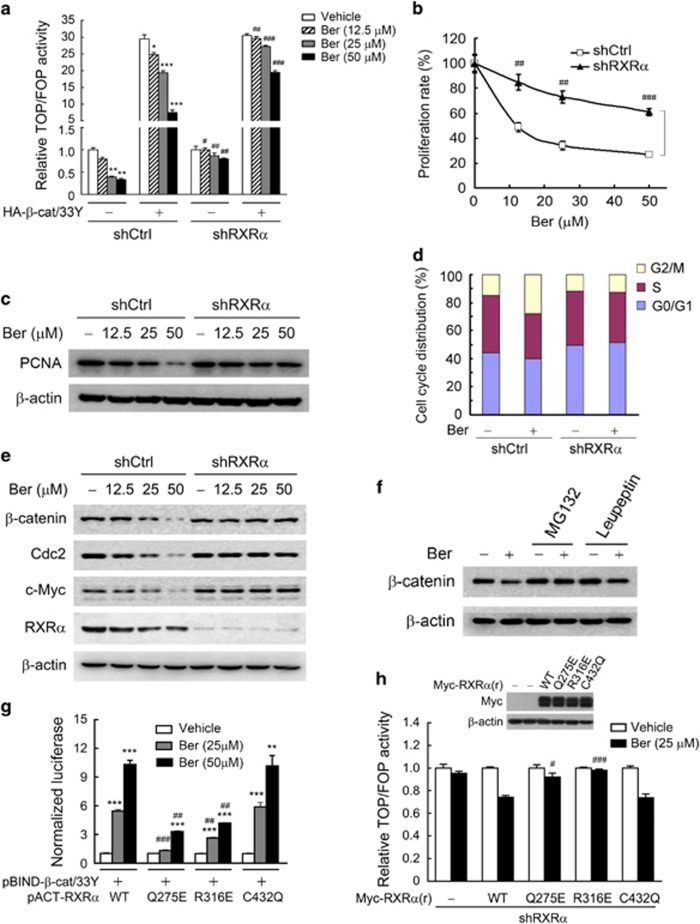
Figure 4.
Binding RXRα was required for berberine to inhibit β-catenin signaling and cell proliferation in colon cancer cells. (a) RXRα mediated the effect of berberine on β-catenin signaling activity as detected by TOP/FOP luciferase assay. KM12C sublines, shCtrl and shRXRα, were cotransfected with pRL-TK, TOPflash or FOPflash plasmid, and HA-β-catenin/33Y or pCMV5-HA vector, and then treated with berberine for 15 h. The base TOP/FOP ratio in each cell sublines was normalized to 1. (b) RXRα-dependent effect of berberine on cell proliferation evaluated by MTT assay. KM12C sublines, shCtrl and shRXRα, were treated with berberine for 15 h. The base proliferation rate in the vehicle-treated group of each cell subline was normalized to 1. (c) Berberine inhibited the expression of PCNA and β-catenin in an RXRα-dependent manner. KM12C cells expressing control or RXRα shRNA were treated with berberine for 15 h, and then subjected to western blot. (d) RXRα mediated the effects of berberine on cell cycle arrest. ShCtrl and shRXRα KM12C cells treated with 50 μm berberine for 15 h were subjected to cell cycle analysis. (e) Berberine inhibited the expression of β-catenin protein and regulates the expression of downstream target genes of β-catenin signaling in an RXRα-dependent manner. (f) Proteasome-dependent effect of berberine on β-catenin protein expression. KM12C cells were pre-incubated with 10 μm MG132 or 25 μg/ml leupeptin for 2 h and then exposed to 50 μm berberine for another 15 h. (g) Binding RXRα was essential for berberine to promote RXRα interaction with β-catenin as detected by mammalian two-hybrid assay. KM12C cells were cotransfected with pBIND-β-catenin/33Y, pACT-RXRα or its mutants and pG5luc vector, and treated with berberine for 15 h. The basal level of transcriptional activity in the vehicle-treated group of RXRα or its mutants was normalized to 1. (h) Binding RXRα was essential for berberine to inhibit the β-catenin signaling activity. KM12C shRXRα cells were transfected with pRL-TK, TOPflash or FOPflash plasmid, and pCMV5-Myc vector or Myc-RXRα(r) and its mutants (the RXRα rescue construct RXRα(r) did not require silence mutation as shRXRα targeted the 3′-UTR region of RXRα mRNA). Twenty-four hours after transfection, cells were treated with 25 μm berberine for 15 h, and then subjected to TOP/FOP luciferase assay. Insets showed the expression levels of Myc-RXRα(r) and its mutants, detected by western blot with anti-Myc antibody. The basal TOP/FOP ratio in each vehicle-treated group was normalized to 1. All data were presented as the mean±s.e.m. of three independent experiments. Significant differences compared with vehicle control of the same HA-β-catenin/33Y or pCMV5-HA vector (a) or RXRα construct (g) were indicated as *P<0.05, **P<0.01 and ***P<0.001. Significant differences of shRXRα vs shCtrl with the same HA-β-catenin/33Y or pCMV5-HA vector (a) or RXRα mutants vs WT (g, h) at the same dose of berberine were indicated as #P<0.05, ##P<0.01 and ###P<0.001. Abbreviation: PCNA, proliferating cell nuclear antigen.