Fig. 5.
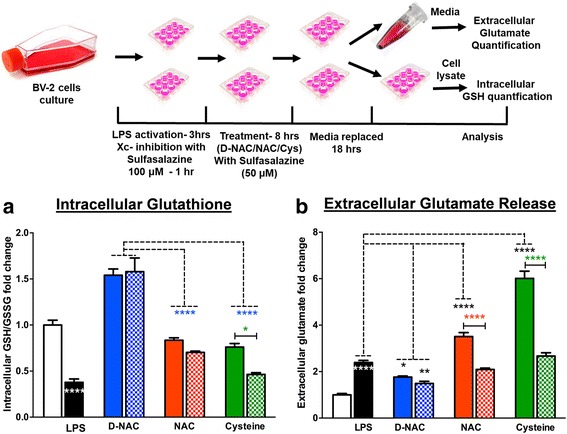
Role of Xc− in NAC internalization in BV2 cells. Mecp2-null and WT brains were acquired, and cells were dissociated and plated to grow. Once confluent, cells were reseeded and treated with LPS (3 h) and sulfasalazine (1 h) and then incubated in cysteine, NAC, or D-NAC with sulfasalazine for 8 h. Then, the cells were incubated in fresh media for 18 h. Cells and media were then taken and used to assess intracellular glutathione levels and extracellular glutamate release. To assess the mechanism of internalization of D-NAC as compared to NAC and cysteine, extracellular glutamate and intracellular glutathione levels were assessed in LPS-activated BV2 cells after treatment when Xc− was functional (without sulfasalazine (solid bars)) and when it was blocked with an Xc− inhibitor (with sulfasalazine (cross-hatched bars)). a In D-NAC-treated cells, GSH increased regardless of Xc− functionality/blockade (blue bars), suggesting that D-NAC bypasses Xc− to exert its anti-oxidant effect intracellularly. In NAC- and cysteine-treated cells, Xc− blockade resulted in a decrease in GSH production, suggesting that NAC and cysteine were internalized in large part via Xc−. b Glutamate levels increased with LPS administration. When Xc− was functional, NAC (red bars) and cysteine (green bars) showed increased glutamate levels, but when Xc− was blocked, glutamate release decreased in NAC- and cysteine-treated samples. In contrast, regardless of Xc− functionality/blockade, D-NAC (blue bars) was effective in reducing glutamate release (*p < 0.05; **p < 0.01; ***p < 0.001; white asterisks denote the comparison between resting and LPS conditions; the black asterisks denote the comparison between LPS-stimulated and LPS + D-NC, NAC, or cysteine conditions; the blue asterisks denote the comparison between LPS + D-NAC and LPS + NAC and LPS + cysteine conditions; the red and green asterisks denote the comparison between LPS + NAC and LPS + NAC + sulfasalazine and LPS + cysteine and LPS + cysteine + sulfasalazine)
