Fig. 5.
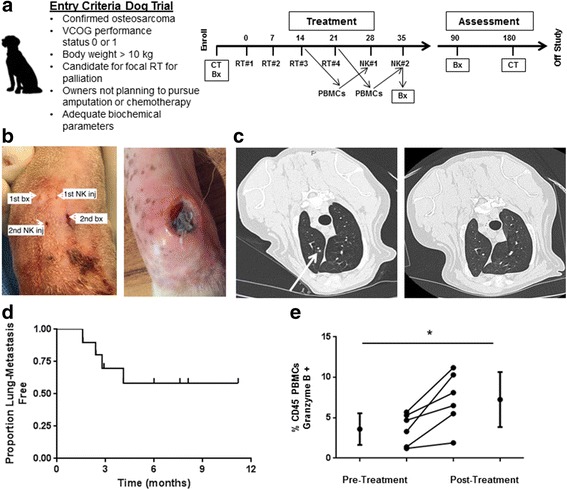
Toxicity and Outcomes in First-in-Dog Clinical Trial of Palliative RT and Intra-tumoral NK for Osteosarcoma. a. Graphic depicts inclusion criteria and schema for clinical trial. During the last 2 weeks of RT, peripheral blood was withdrawn for isolation, expansion, and activation of NK cells. b. Infection at NK injection site was observed in 3 dogs (Table 2), and management of these infections required surgical debridement in 2 dogs. (Left) Per protocol, we placed the needle insertion sites in distinct locations for the biopsy and NK injection procedures, and interestingly, the injection site infections occurred at the NK injection site (Right). c. Of 10 dogs with OSA treated with focal RT and autologous NK transfer who reached the 6-month primary endpoint, 5 remained metastasis-free, including resolution of suspicious pulmonary nodules in one patient. (Left) At 3 month follow up, chest computed tomography depicts 3 mm pulmonary nodule (arrow) that was interpreted as highly suspicious for metastatic sarcoma. At the 6-month time point, this suspicious pulmonary nodule had completely resolved (Right). d. Kaplan-Meier analysis shows the pulmonary metastasis-free survival for our clinical trial cohort. We observed a 50% proportion of lung metastases with palliative RT and autologous NK transfer (P < 0.05 compared to historical controls using a 1-sided test and a type I error rate of 5%). e. Analysis of PBMCs from our clinical trial patients demonstrated a significant increase in Granzyme B + CD45+ cells (3.6 ± 1.9% to 7.3 ± 3.4%) from baseline to post-treatment. *P < 0.05 via paired Student’s T-test
