Abstract
Purpose
Avoiding chemotherapy during the last 30 days of life has become a goal of cancer care in the United States and Europe, yet end-of-life chemotherapy administration remains a common practice worldwide. The purpose of this study was to determine the frequency of and factors predicting end-of-life chemotherapy administration in Uganda.
Methods
Retrospective chart review and surveys and interviews of providers were performed at the Uganda Cancer Institute (UCI), the only comprehensive cancer center in the area, which serves a catchment area of greater than 100 million people. All adult patients at the UCI with reported cancer deaths between January 1, 2014, and August 31, 2015 were included. All UCI physicians were offered a survey, and a subset of physicians were also individually interviewed.
Results
Three hundred ninety-two patients (65.9%) received chemotherapy. Age less than 55 years (odds ratio [OR], 2.30; P = .004), a cancer diagnosis greater than 60 days before death (OR, 9.13; P < .001), and a presenting Eastern Cooperative Oncology Group performance status of 0 to 2 (OR, 2.47; P = .001) were associated with the administration of chemotherapy. More than 45% of patients received chemotherapy in the last 30 days of life. No clinical factors were predictive of chemotherapy use in the last 30 days of life, although doctors reported using performance status, cancer stage, and tumor chemotherapy sensitivity to determine when to administer chemotherapy. Patient expectations and a lack of outcomes data were important nonclinical factors influencing chemotherapy administration.
Conclusion
Chemotherapy is administered to a high proportion of patients with terminal cancer in Uganda, raising concern about efficacy. Late presentation of cancer in Uganda complicates end-of-life chemotherapy recommendations, necessitating guidelines specific to sub-Saharan Africa.
INTRODUCTION
Although cytotoxic chemotherapy is often associated with improvements in quality and quantity of life among patients with advanced cancer,1-3 the benefit of its use at the end of life is uncertain. Chemotherapy in the last days of life is not associated with a survival benefit, and recent data suggest it may cause harm by decreasing quality of life and increasing costs.4-6 As a result, death within 30 days of chemotherapy has been used as a quality indicator for cancer care.6,7 Both the European Society of Medical Oncology and ASCO have published position statements encouraging discussions about the appropriate cessation of chemotherapy.8,9 However, the implementation of such recommendations has been limited.10,11
In 2012 alone, there were an estimated 645,000 new cancer diagnoses and more than 450,000 cancer-related deaths in Africa.12 In Uganda, cancer survival is less than 13% for all cancers except for breast, and of the nearly 4,000 new patients with cancer seen annually at the Uganda Cancer Institute (UCI), more than 75% present with stage III or IV disease (Low et al, manuscript in preparation).13 Although various prognostic indices aid in the decision of whether and when to administer chemotherapy in resource-abundant areas, the ability to accurately predict response to therapy and prognosis in Uganda is limited by incomplete laboratory and clinical data. Deciding whether to administer chemotherapy in advanced disease in this setting and, if administered, determining the optimal time to stop chemotherapy are challenging. To address the dearth in knowledge concerning chemotherapy use at the end of life in sub-Saharan Africa and to better gauge current oncology practices at the end of life in Uganda, we conducted a mixed-methods study to evaluate the use of chemotherapy at the UCI. The UCI employs 28 doctors and is the only comprehensive cancer center in the area, serving a catchment area of more than 100 million people in Uganda and adjacent regions of several neighboring countries (Uganda Cancer Institute/Hutchinson Cancer Care Alliance, unpublished data).
METHODS
Chart Abstraction
Medical records of all patients ≥ 18 years old diagnosed with cancer at the UCI who died between January 1, 2014, and August 31, 2015, were reviewed. Data abstracted from eligible participants’ charts included demographic information, clinical diagnosis, date of diagnosis, stage of disease, chemotherapy administration, date of last chemotherapy, performance status at time of chemotherapy (or performance status at admission if chemotherapy was never administered), comorbidities, involvement of a palliative care specialist, and date of death.
We defined chemotherapy as cytotoxic agents (eg, cisplatin), targeted therapy (eg, imatinib), and immunotherapy (eg, rituximab). Endocrine (including leuprolide) and bisphosphonate therapies were defined as supportive treatments. Cancers with a historic response rate of at least 50% to first-line chemotherapy were deemed chemotherapy sensitive; all other tumors were considered to be not chemotherapy sensitive.6,14,15 Cancer types that were not specified in the medical charts were excluded from the chemotherapy sensitivity analysis. Early diagnosis was defined as being diagnosed with cancer more than 60 days before death.16 Patients whose medical files either had no confirmatory cancer diagnosis or could not be found by the medical records office were excluded. Vital status was determined by the UCI medical records office and confirmed by each patient file. Only patients who were confirmed to be deceased were included; patients who died at home with no verification by the UCI records office were excluded.
Survey and Interview
After discussion with UCI physicians and adaptations from the literature, a survey was designed that assessed physician views on end-of-life chemotherapy and factors influencing the cessation of chemotherapy.17-19 This survey was offered to all doctors at the UCI, excluding the authors, who had provided clinical care within 6 months of survey administration.
Between April and May 2016, one author (D.L.) conducted one-on-one semistructured interviews with seven doctors from the UCI who were identified via purposive sampling to capture diverse perspectives. Participant recruitment continued until thematic saturation was reached. An interview guide with open-ended questions was used to explore participants’ management approach to advanced cancers. Open-ended questions were followed with probes for in-depth understanding of participants’ responses. Finally, participants were asked to assess and give care plans for two hypothetical patients with metastatic cancer (Data Supplement). These interviews were conducted in English, audio-recorded, and transcribed verbatim by the author (D.L.).
Data Analysis
REDCap software (projectredcap.org) captured the chart abstraction and survey data. STATA version 14.1 (STATA, College Station, TX) was used for statistical analysis of the chart review. Bivariable logistic regression was used to test for factors correlated with the receipt of chemotherapy (because life expectancy after cancer diagnosis was so limited in this cohort) and factors influencing chemotherapy administration in the last 30 days of life. All bivariable factors with a P ≤ .20 were included in a multivariable logistic regression.20 Proportional statistics were used to describe survey results regarding the frequency with which doctors felt certain factors influenced their chemotherapy decision making.
For the individual interviews, two authors (D.L. and E.C.M.) conducted qualitative thematic content analysis with the transcribed interviews to develop and iteratively modify a codebook. Each code was reviewed for internal consistency and described with a specific definition to ensure a standard definition by each researcher. Data analysis was done concurrently with data collection in an iterative manner to ensure saturation was reached. Atlas.ti v1.0.46 (ATLAS.ti Scientific Software Development, Berlin, Germany) was used for coding organization and analysis. This study was approved by the Fred Hutchinson Cancer Research Center Review Board (Federalwide Assurance [FWA] No. 00001920), the Uganda Cancer Institute Research Ethics Committee (FWA No. 00021897), and the Uganda National Council on Science and Technology (FWA No. 00001293).
RESULTS
Chart Review
A total of 738 adult patients at the UCI met eligibility criteria. For these patients, 138 medical charts (18.7%) could not be found, and five charts (0.7%) were created but had no confirmed cancer diagnosis, leaving 595 medical charts (80.6%) for this analysis.
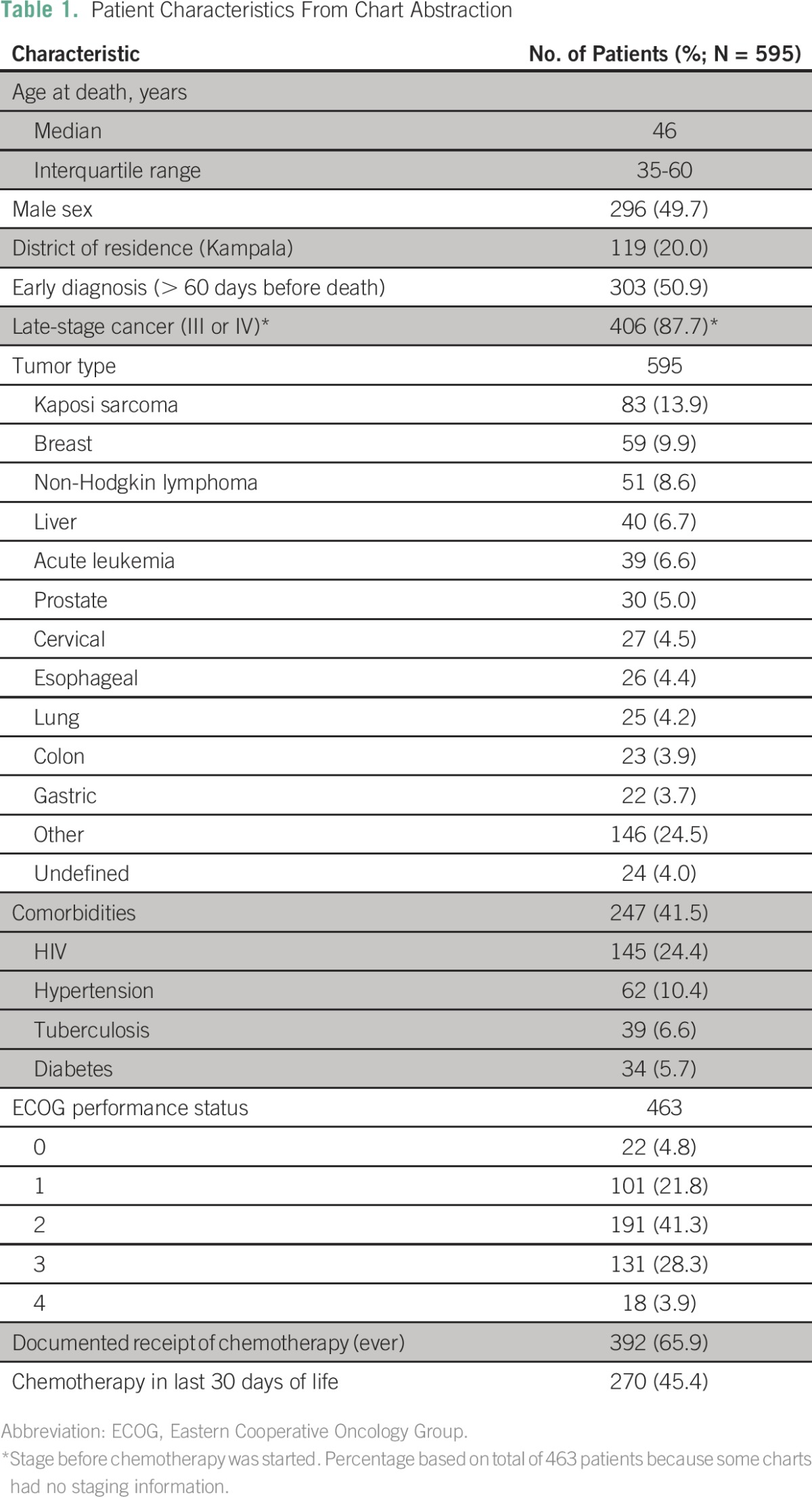
The median patient age was 46 years (interquartile range, 35 to 60 years; range, 18 to 95 years), and men and women were equally represented (Table 1). Patients were clinically diagnosed with cancer a median of 57 days before their date of death (interquartile range, 13 to 225 days).
Table 1.
Patient Characteristics From Chart Abstraction
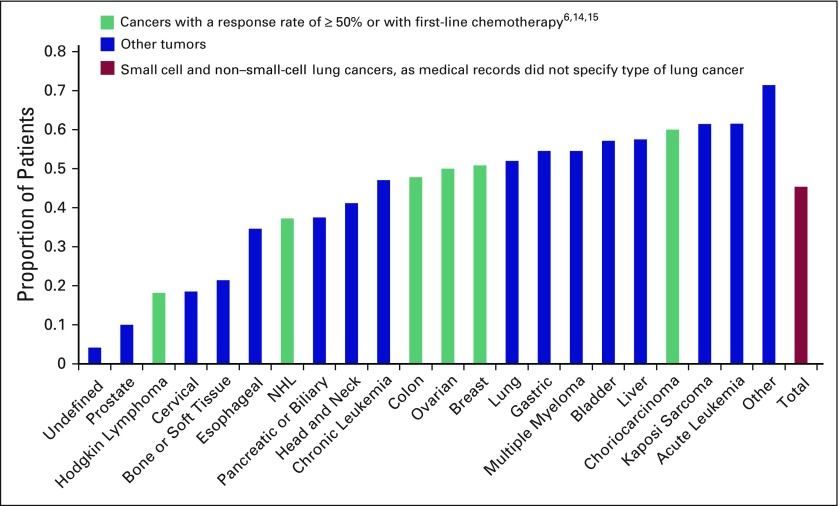
The receipt and timing of chemotherapy varied by cancer type (Fig 1). Three hundred ninety-two patients (65.9%) received any chemotherapy. In total, 45.4% of patients received chemotherapy in the last 30 days of life. Of those ever receiving chemotherapy, 68.9% received chemotherapy in the last 30 days of life.
Fig 1.
Proportion of patients treated in last 30 days of life by cancer type among all deceased patients (N = 595). NHL, non-Hodgkin lymphoma.
The receipt of chemotherapy was associated with age, timing of cancer diagnosis, and Eastern Cooperative Oncology Group (ECOG) performance status at presentation (Table 2). Patients younger than age 55 years (odds ratio [OR], 2.30; P = .004), those who were diagnosed with cancer more than 60 days before death (OR, 9.13; P < .001), and those with presenting ECOG performance statuses of 0 to 2 (OR, 2.47; P = .001) were more likely to receive chemotherapy (Table 2).
Table 2.
Factors Associated With Receipt of Chemotherapy and Chemotherapy Within 30 Days of Death in 595 Patients
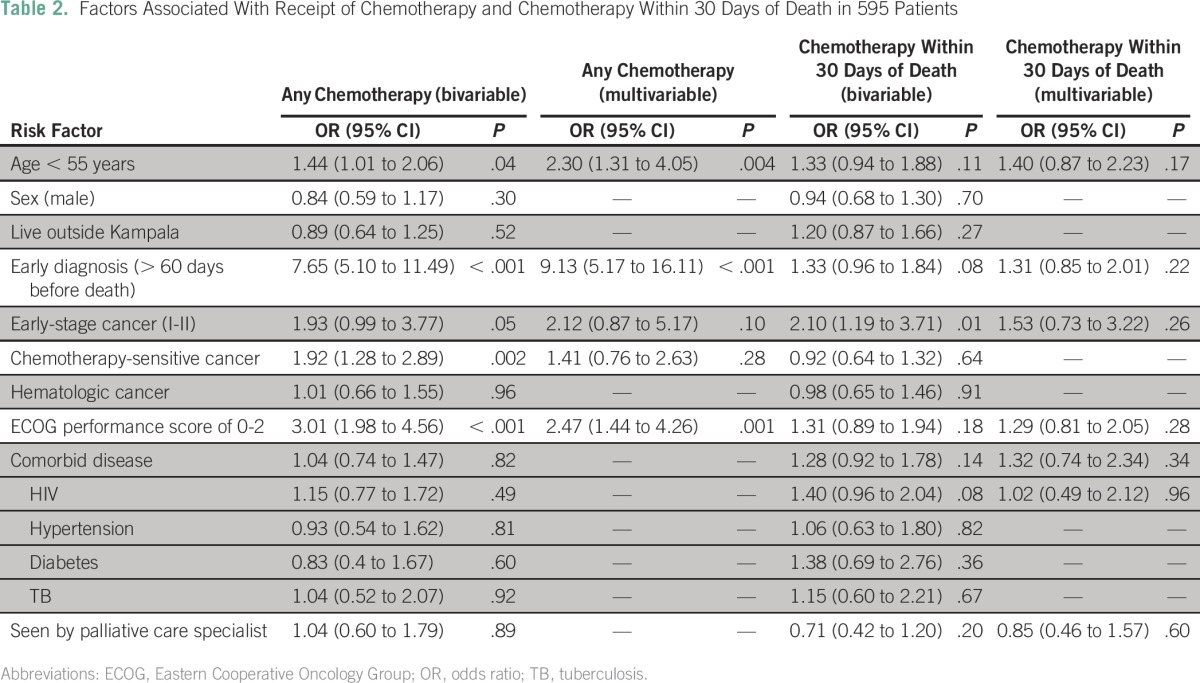
Age, timing of cancer diagnosis, and performance status were not predictive of receiving chemotherapy in the last 30 days of life. There were no observed factors, including patient residence, comorbidities, or the involvement of palliative care specialists, that were predictive of chemotherapy administration in the last 30 days of life (Table 2).
Survey and Interviews
Twenty-five of 26 eligible doctors at the UCI participated in the survey (Table 3). Eighteen doctors (72%) were men, and 22 doctors (88%) reported receiving training in palliative care during their medical education (Table 3).
Table 3.
Physician Characteristics
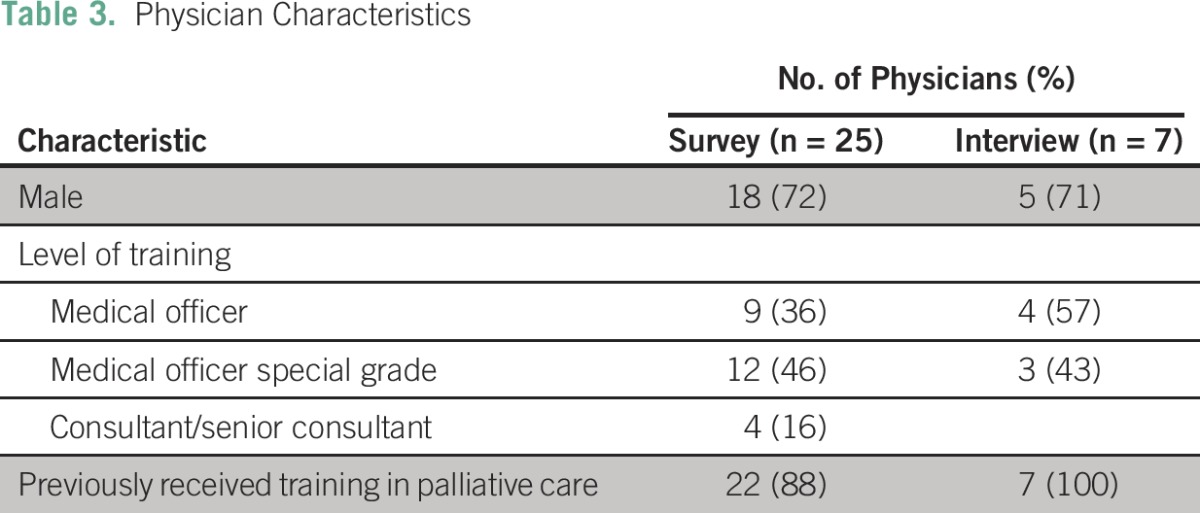
Seven of the surveyed doctors also participated in individual interviews (Table 3). They included medical officers (completed medical school) and medical officers special grade (completed medical school and masters-level training; Table 3).
Factors Influencing End-of-Life Chemotherapy Use
UCI physicians referenced several factors influencing their decision to use chemotherapy at the end of life, including performance status, cancer type and chemotherapy sensitivity, patient expectations, and a lack of treatment guidelines. All doctors interviewed emphasized the difficulty in making decisions regarding end-of-life chemotherapy.
Performance Status
When deciding whether to give chemotherapy in advanced cancers, six of seven interviewed doctors referenced the importance of assessing the patient’s ECOG performance status.
What is the patient able to do for themselves? Are they ECOG 1, ECOG 2, or are they ECOG 3 or 4? Studies have shown that with ECOG 3 or 4 and with advanced disease, outcomes are not good. And so, again that would guide me, should I give chemo, should I not give chemo. (Doctor 6)
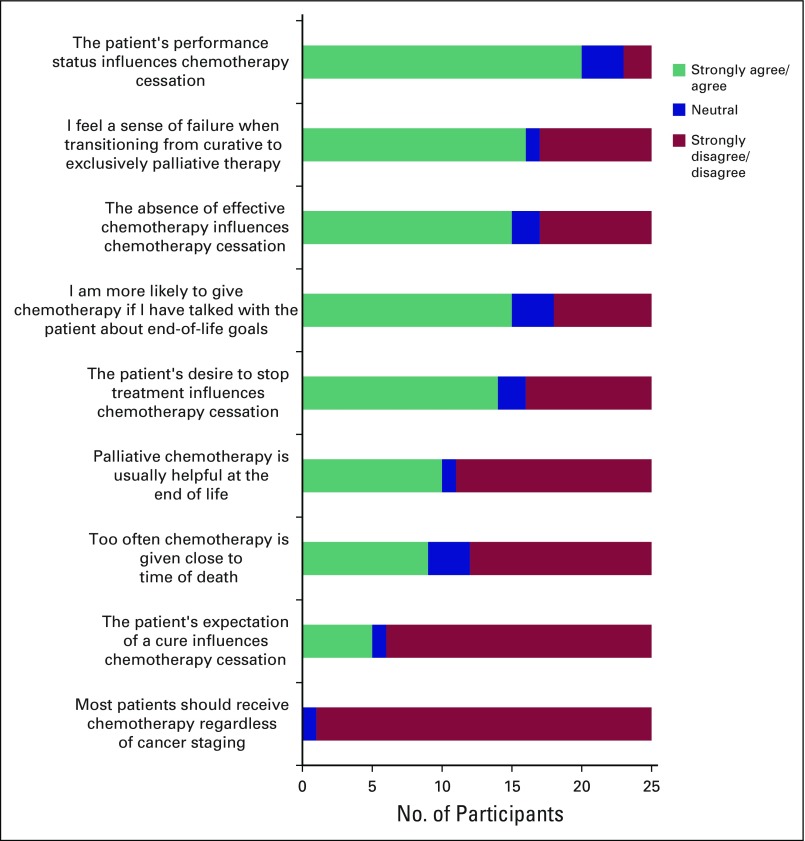
The survey reinforced this statement, because 80% of doctors noted that performance status strongly influenced their decision to terminate chemotherapy (Fig 2).
Fig 2.
Physician survey results on factors influencing cessation of chemotherapy and attitudes toward end-of-life chemotherapy.
Type of Cancer
In determining chemotherapy treatment, the doctors who were interviewed also stressed the importance of cancer type.
There are some cancers that respond to chemo even when they are stage 4 and there are those that don’t respond. (Doctor 6)
Someone might come in severely distressed with [lymphoma and] superior vena cava obstruction, desaturating even on oxygen and they are very, very sick. But I know I put in 1 cycle of chemotherapy and within two days all of that will have gone away and they feel much better. Versus someone who comes in with 6-month history of osteosarcoma with mets in the lung, in the liver and stuff like that . . . so for those two patients those decisions are made differently. (Doctor 3)
Expectation of Treatment
Beyond clinical information, interviewed doctors highlighted the importance of patients’ expectations; doctors mentioned sometimes administering treatment to satisfy the patient.
I have not yet seen a patient who comes in with advanced disease and doesn’t want chemotherapy. (Doctor 4)
We live in an environment where there is a lot of stress or demand from the family. Once a patient comes, they expect treatment and bouncing them back home—they feel really that it’s been very, very unfair to not get any treatment. (Doctor 7)
Lack of Data and Guidelines
Interviewed doctors repeatedly referenced the lack of definitive treatment guidelines as a challenge in clinical decision making.
There’s no general protocol used per se. As you realize when you see these patients, they’re all kind of unique . . . so I won’t lie to you that we have a general protocol where anyone with stage 4 cancer, do this, A, B and C. No. (Doctor 3)
I think here at the cancer institute, it’s a case-by-case scenario. Because most of our patients come with advanced disease, ok? They are often in very poor performance status, ok? So to say, if it’s a 3 or a 4 don’t treat, would be like not treating 95% of the patients. So I think it’s a case-by-case scenario. (Doctor 1)
Because doctors do not have end-of-life treatment protocols, they cannot use evidence-based criteria to decide when to treat. In a hypothetical clinical scenario involving metastatic colorectal cancer presented to six of the seven interviewees, two of six doctors claimed they would treat with chemotherapy, two of six claimed they would not treat with chemotherapy, and two of six were unsure.
Chemotherapy Culture
Although many factors influence physicians’ decisions about whether to treat advanced cancers with chemotherapy, doctors felt that it was best to treat when there was uncertainty. Doctors reported positive feelings toward chemotherapy use, noting it offered the possibility of cure or at least symptom relief.
Default Practice of Treatment
When clinical presentations were not clear-cut, most doctors reported defaulting to treating with chemotherapy.
We just give chemotherapy because yes it’s there and I know how to write it. That’s what I really feel. . . . As a doctor you do what you know best—how to prescribe drugs. (Doctor 2)
There was no consensus on whether the treatment patterns at the UCI were best clinical care or overtreatment. In the survey, 36% of physicians felt that chemotherapy was given too close to death, 12% were unsure, and 52% disagreed that chemotherapy was given too close to death (Fig 2). Of the interviewed doctors who felt chemotherapy was given too close to death, there was strong concern over the aggressive administration of chemotherapy.
It’s horrible. Our outcomes, our treatment outcomes are terrible. I don’t have figures or numbers, but I would think we are not doing much. We are killing more than we are curing . . . patients are given chemotherapy and then in 48 hours they are dead. You know? And you’re asking yourself, why? (Doctor 1)
Hope
Doctors tended to treat patients because they felt there was always a chance for a cure and that doctors should not take hope away from patients.
Everyone deserves a chance to, you know, try for a cure. Like I give you and maybe you improve. . . . People don’t want to give up on their patients. (Doctor 1)
In the survey, 16 (67%) of 24 doctors reported feeling a sense of failure when transitioning from curative therapy to exclusively palliative treatment (Fig 2).
DISCUSSION
End-of-life cancer care is a significant challenge in sub-Saharan Africa because the best timing and use of chemotherapy in advanced cancers are unclear. We studied end-of-life care at the UCI, reviewing all recorded deaths among adult patients with cancer over a 20-month time period, as well as surveying and interviewing doctors at the UCI. We aimed to describe end-of-life chemotherapy practices and assess the culture of chemotherapy use in patients with terminal cancer in Uganda.
In our study, patients younger than age 55 years, those with a better performance status, and those diagnosed with cancer more than 60 days before death were more likely to receive chemotherapy. Nearly half of all patients were given chemotherapy in the last 30 days of life. Despite doctors’ insistence in the survey and interviews that they used performance status, cancer type, and tumor chemotherapy sensitivity in deciding whether to give chemotherapy to terminal patients, none of these factors were found to be predictive of chemotherapy use in the last 30 days of life. Our data suggest that chemotherapy treatment in patients with advanced cancer at the UCI may be influenced more strongly by nonclinical factors than by clinical factors.
The frequency of chemotherapy administration near the end of life in Uganda is one of the highest in the world.16 There are many possible explanations for this. First, uncertainties in cancer staging and the absence of prognostic biomarkers may have led providers to err on the side of treating more aggressively. This is suggested by the qualitative data, where doctors emphasized treating with chemotherapy when there was clinical uncertainty. Second, more than half of UCI patients were diagnosed with cancer less than 2 months before death. All of these patients were chemotherapy naïve, and so such treatment often may have been appropriate. Unlike many other studies documenting the use of chemotherapy near the end of life with patients treated for relapsed and refractory disease, the patients in our cohort were predominantly receiving first-line chemotherapy. Third, the distribution of cancer types in our cohort varied substantially from other published studies. Kaposi sarcoma, the most common cancer in our study, was one of the most commonly treated cancers within the last month of life and has rarely been reported in previous studies. Other studies have found end-of-life chemotherapy treatment to be correlated with cancer type, and this may partially explain the high rates of chemotherapy use in Uganda.21-23 Finally, cultural expectations around cancer care, both in patients and providers, may differ substantially from settings where chemotherapy use at the end of life has previously been studied. Accessible and affordable chemotherapy has only recently been available to many patients in Uganda. Because it often takes patients more than 7 months from the onset of cancer symptoms to register at the UCI (Low et al, manuscript in preparation), many patients and families consume nearly all of their resources to obtain a cancer diagnosis and subsequently feel entitled to treatment upon arriving at the UCI.
Regardless of rationale, the high rate of chemotherapy use at the end of life raises questions of efficacy. It is difficult to gauge the appropriateness of chemotherapy use at the end of life in Uganda because of the dynamic clinical picture in which late-stage, treatment-naïve patients are the norm. This suggests there may be opportunities to develop evidence-based guidelines for the provision of chemotherapy in patients with advanced-stage cancers in Uganda. Such guidelines should be informed by clinical criteria and nonclinical factors that influence doctors’ decisions. Although UCI doctors reported making end-of-life chemotherapy decisions on the basis of ECOG performance status, cancer type, and patient hopes, as has been noted in other studies throughout the world,6,24-35 the chart abstraction showed that none of these factors predicted chemotherapy use in the last 30 days of life. This difference in perceived and observed behavior demonstrates that these clinical measures are not the only decision-altering factors. The other key factors identified in the interviews, including a lack of outcomes data and patients’ expectations of treatment, may influence doctors’ decisions more than previously appreciated. Hence, although it remains essential to develop evidence-based end-of-life treatment guidelines relevant to sub-Saharan Africa, such clinical algorithms must understand and incorporate nonclinical factors into their provisions for chemotherapy treatment near the end of life.
It is important to acknowledge this study’s limitations. A significant amount of data was missing; more than 18% of medical files were not found. Additionally, although deaths on the inpatient units were reliably recorded in the medical records logbook, deaths outside of the hospital were only recorded and included if family of deceased patients contacted the UCI about a patient’s death. This biases the data to in-hospital deaths and, therefore, may overstate the use of chemotherapy near death. Finally, the medical records inconsistently recorded chemotherapy doses and rarely listed cause of death, making it difficult to interpret the efficacy of chemotherapy administration.
In conclusion, as the first published examination of chemotherapy use at the end of life in sub-Saharan Africa to our knowledge, this study showed that the rate of chemotherapy use in the last 30 days of life in Uganda is among the highest in the world. This is likely a result of myriad factors including late presentation of disease by chemotherapy-naïve patients. Although age, timing of diagnosis, and performance status were predictive of the use of chemotherapy as a treatment modality, there were no predictors of chemotherapy use in the last 30 days of life, despite doctors’ qualitative insistence on using performance status, cancer staging, and cancer type to make these decisions. Because aggressive, end-of-life chemotherapy has demonstrated no survival benefit and often causes harm in resource-abundant settings,5,7 there is need for evidence-based guidelines for end-of-life chemotherapy treatment specific to sub-Saharan Africa.
Footnotes
Supported by National Institutes of Health (NIH) Research Training Grant No. R25 TW009345 awarded to the Northern Pacific Global Health Fellows Program by the Fogarty International Center, as well as NIH Grants No. P30 CA015704, P30 AI027757, D43 CA153720, and U54 CA190146. The use of REDCap software was supported by Grant No. UL1TR000423 from the National Center for Research Resources and NIH.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel Low, Henry Ddungu, Elizabeth Namukwaya, Mhoira Leng, Corey Casper
Financial support: Corey Casper
Administrative support: Corey Casper
Collection and assembly of data: Daniel Low
Data analysis and interpretation: Daniel Low, Emily C. Merkel, Manoj Menon, Gary H. Lyman, Mhoira Leng, Corey Casper
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Daniel Low
No relationship to disclose
Emily C. Merkel
No relationship to disclose
Manoj Menon
No relationship to disclose
Gary H. Lyman
No relationship to disclose
Henry Ddungu
No relationship to disclose
Elizabeth Namukwaya
No relationship to disclose
Mhoira Leng
No relationship to disclose
Corey Casper
Consulting or Advisory Role: Temptime
Research Funding: Janssen Pharmaceuticals
Travel, Accommodations, Expenses: GlaxoSmithKline
REFERENCES
- 1.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial J Clin Oncol 152403-2413, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial J Clin Oncol 3123-29, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Cullen MH, Billingham LJ, Woodroffe CM, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: Effects on survival and quality of life J Clin Oncol 173188-3194, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life JAMA Oncol 1778-784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito AM, Landrum MB, Neville BA, et al. The effect on survival of continuing chemotherapy to near death. BMC Palliat Care. 2011;10:14. doi: 10.1186/1472-684X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zdenkowski N, Cavenagh J, Ku YC, et al. Administration of chemotherapy with palliative intent in the last 30 days of life: The balance between palliation and chemotherapy Intern Med J 431191-1198, 2013 [DOI] [PubMed] [Google Scholar]
- 7.O’Brien ME, Borthwick A, Rigg A, et al. Mortality within 30 days of chemotherapy: A clinical governance benchmarking issue for oncology patients Br J Cancer 951632-1636, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherny NI, Catane R, Kosmidis P.ESMO takes a stand on supportive and palliative care Ann Oncol 141335-1337, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Peppercorn JM, Smith TJ, Helft PR, et al. American Society of Clinical Oncology statement: Toward individualized care for patients with advanced cancer J Clin Oncol 29755-760, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life J Clin Oncol 22315-321, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Temel JS, McCannon J, Greer JA, et al. Aggressiveness of care in a prospective cohort of patients with advanced NSCLC Cancer 113826-833, 2008 [DOI] [PubMed] [Google Scholar]
- 12. International Agency for Research on Cancer: GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 13.Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: A population-based study Lancet Oncol 11165-173, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Näppä U, Lindqvist O, Rasmussen BH, et al. Palliative chemotherapy during the last month of life Ann Oncol 222375-2380, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Emanuel EJ, Young-Xu Y, Levinsky NG, et al. Chemotherapy use among Medicare beneficiaries at the end of life Ann Intern Med 138639-643, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Sezgin Goksu S, Gunduz S, Unal D, et al. Use of chemotherapy at the end of life in Turkey. BMC Palliat Care. 2014;13:51. doi: 10.1186/1472-684X-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentlandt K, Krzyzanowska MK, Swami N, et al. Referral practices of oncologists to specialized palliative care J Clin Oncol 304380-4386, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Hilden JM, Emanuel EJ, Fairclough DL, et al. Attitudes and practices among pediatric oncologists regarding end-of-life care: Results of the 1998 American Society of Clinical Oncology survey J Clin Oncol 19205-212, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ward AM, Agar M, Koczwara B.Collaborating or co-existing: a survey of attitudes of medical oncologists toward specialist palliative care Palliat Med 23698-707, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonçalves JF, Goyanes C.Use of chemotherapy at the end of life in a Portuguese oncology center Support Care Cancer 16321-327, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Braga S, Miranda A, Fonseca R, et al. The aggressiveness of cancer care in the last three months of life: A retrospective single centre analysis Psychooncology 16863-868, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Ho TH, Barbera L, Saskin R, et al. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada J Clin Oncol 291587-1591, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bluhm MV: Factors influencing oncologists’ use of chemotherapy in patients at the end of life: A qualitative study. https://deepblue.lib.umich.edu/bitstream/handle/2027.42/84635/mbluhm_1.pdf?sequence=1. [Google Scholar]
- 25.de Kort SJ, Pols J, Richel DJ, et al. Understanding palliative cancer chemotherapy: About shared decisions and shared trajectories Health Care Anal 18164-174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough L, McKinlay E, Barthow C, et al. A model of treatment decision making when patients have advanced cancer: How do cancer treatment doctors and nurses contribute to the process? Eur J Cancer Care (Engl) 19482-491, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Harrington SE, Smith TJ.The role of chemotherapy at the end of life: “When is enough, enough?” JAMA 2992667-2678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarenmalm EK, Thorén-Jönsson A-L, Gaston-Johansson F, et al. Making sense of living under the shadow of death: Adjusting to a recurrent breast cancer illness Qual Health Res 19116-130, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Schildmann J, Tan J, Salloch S, et al. “Well, I think there is great variation...”: A qualitative study of oncologists’ experiences and views regarding medical criteria and other factors relevant to treatment decisions in advanced cancer Oncologist 1890-96, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buiting HM, Rurup ML, Wijsbek H, et al. Understanding provision of chemotherapy to patients with end stage cancer: Qualitative interview study. BMJ. 2011;342:d1933. doi: 10.1136/bmj.d1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto K, Yonemori K, Katsumata N, et al. Factors that affect the duration of the interval between the completion of palliative chemotherapy and death Oncologist 14752-759, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: Prospective cohort study. BMJ. 2014;348:g1219. doi: 10.1136/bmj.g1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chouliara Z, Miller M, Stott D, et al. Older people with cancer: Perceptions and feelings about information, decision-making and treatment—A pilot study Eur J Oncol Nurs 8257-261, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Liu TW, Chang WC, Wang HM, et al. Use of chemotherapy at the end of life among Taiwanese cancer decedents, 2001-2006 Acta Oncol 51505-511, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Hui D, Karuturi MS, Tanco KC, et al. Targeted agent use in cancer patients at the end of life J Pain Symptom Manage 461-8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]