Abstract
Logging to “salvage” economic returns from forests affected by natural disturbances has become increasingly prevalent globally. Despite potential negative effects on biodiversity, salvage logging is often conducted, even in areas otherwise excluded from logging and reserved for nature conservation, inter alia because strategic priorities for post-disturbance management are widely lacking.
A review of the existing literature revealed that most studies investigating the effects of salvage logging on biodiversity have been conducted less than 5 years following natural disturbances, and focused on non-saproxylic organisms.
A meta-analysis across 24 species groups revealed that salvage logging significantly decreases numbers of species of eight taxonomic groups. Richness of dead wood dependent taxa (i.e. saproxylic organisms) decreased more strongly than richness of non-saproxylic taxa. In contrast, taxonomic groups typically associated with open habitats increased in the number of species after salvage logging.
By analysing 134 original species abundance matrices, we demonstrate that salvage logging significantly alters community composition in 7 of 17 species groups, particularly affecting saproxylic assemblages.
Synthesis and applications. Our results suggest that salvage logging is not consistent with the management objectives of protected areas. Substantial changes, such as the retention of dead wood in naturally disturbed forests, are needed to support biodiversity. Future research should investigate the amount and spatio-temporal distribution of retained dead wood needed to maintain all components of biodiversity.
Keywords: bark beetle, climate change, dead wood, disturbed forest, fire, natural disturbance, post-disturbance logging, salvage logging, saproxylic taxa, windstorm
1. Introduction
The frequency and extent of stand-replacing natural disturbances, such as wildfires, windstorms and insect outbreaks, has increased considerably during recent decades, particularly in the Northern Hemisphere (Kurz et al., 2008; Seidl, Schelhaas, Rammer, & Verkerk, 2014). Natural disturbances can enhance the structural heterogeneity of forests, create habitats for species-rich assemblages of high conservation value and increase the long-term resilience of forests to future stressors (Swanson et al., 2011). However, societal demand for timber and/or pest reduction compels forest managers to “salvage” timber by logging before it deteriorates, a common practice even in locations otherwise exempt from conventional green-tree harvesting, such as national parks or wilderness areas (Figure 1) (Chylarecki & Selva, 2016; Thorn et al., 2014). Such salvage logging reduces the amount of dead wood, alters successional trajectories, affects biodiversity, and can influence restoration costs and subsequent fire hazards (Lindenmayer, Burton, & Franklin, 2008; Waldron, Ruel, & Gauthier, 2013). Consequently, conflicts often emerge between natural resource managers, policy-makers and conservationists on how to handle naturally disturbed forests (González & Veblen, 2007; Lindenmayer, Thorn, & Banks, 2017; Lindenmayer et al., 2004; Schmiegelow, Stepnisky, Stambaugh, & Koivula, 2006). This has resulted in intense public debates (Lindenmayer et al., 2017; Nikiforuk, 2011; Stokstad, 2006).
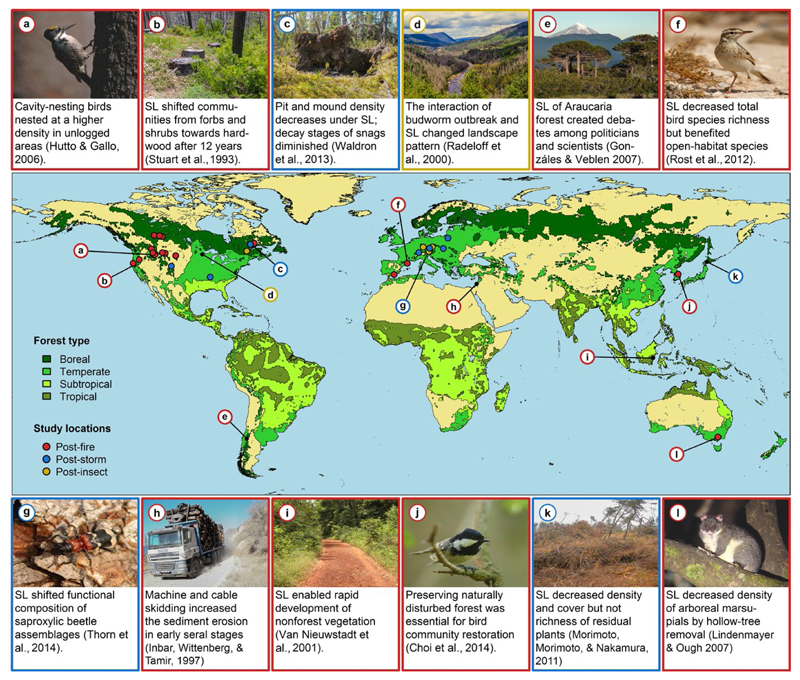
Figure 1.
Salvage logging (SL) is commonly applied after wildfires, windstorms or insect outbreaks, and leads to changes in habitats and community compositions in various forest ecosystems around the world (as highlighted by the studies illustrated in panels (a–l). Study locations (coloured circles) represent study sites that contributed data to our meta-analysis. Photographs by authors.
Different natural disturbance regimes leave distinct types of biological and/or structural legacies (Franklin et al., 2000). For instance, forests killed by wildfire or insect outbreaks are characterized by large numbers of snags, while windstorms create uprooted trees (Swanson et al., 2011). Salvage logging typically removes or alters these legacies. The responses of saproxylic and non-saproxylic species groups to salvage logging thus depend on their relation to (dead wood) legacies affected by salvage logging (Lindenmayer et al., 2008). Consequently, different taxonomic groups in different types of natural disturbances may respond differently to salvage logging (Zmihorski & Durska, 2011). Numerous studies have focused on the effects of salvage logging after natural disturbances on species richness and the community composition of various taxa such as vascular plants (Blair, McBurney, Blanchard, Banks, & Lindenmayer, 2016; Macdonald, 2007; Stuart, Grifantini, Fox, & Fox, 1993), carabids (Cobb, Langor, & Spence, 2007; Koivula & Spence, 2006; Phillips, Cobb, Spence, & Brigham, 2006), birds (Castro, Moreno-Rueda, & Hódar, 2010; Choi, Lee, Nam, Lee, & Lim, 2014; Nappi & Drapeau, 2009; Saab, Russell, & Dudley, 2009; Thorn, Werner, et al., 2016; Zmihorski, 2010), and saproxylic organisms (i.e. those depending on dead wood during some part of their life cycles; Cobb et al., 2011; Norvez, Hébert, Bélanger, Hebert, & Belanger, 2013).
Two main effects of salvage logging on biodiversity arise recurrently from the existing body of literature. First, salvage logging reduces the richness of taxonomic groups or abundance of particular species that depend on dead wood. For instance, salvage logging decreased nesting density of cavity-nesting-birds that usually breed in fire-killed trees (Hutto & Gallo, 2006). Similarly, post-storm logging decreased the total number of saproxylic beetle species and the number of threatened species (Thorn et al., 2014). Second, studies that investigate a set of different taxonomic groups have demonstrated that salvage logging can alter the community composition of both saproxylic and non-saproxylic organisms, while the effects on the overall number of species can be small (Thorn, Bässler, Bernhardt-Römermann, et al., 2016). For instance, post-storm salvage logging in Minnesota greatly diminished bird communities, while fewer differences in the tree cover were detected (Lain, Haney, Burris, & Burton, 2008). However, previous attempts to summarize knowledge on the effects of salvage logging on biodiversity have focused mainly on salvage logging of burned forests (Lindenmayer & Noss, 2006; Lindenmayer et al., 2008; McIver & Starr, 2000; Thorn, Bässler, Svoboda, & Müller, 2016), and a quantitative assessment of salvage logging impacts on biodiversity is still lacking, particularly across different taxonomic groups and in response to different types of disturbances (Figure 1).
Here, we reviewed the scientific literature and compiled existing data to quantify the effects of salvage logging after wildfire, wind-storms and insect outbreaks on (1) species numbers via a meta-analysis of 238 individual comparisons of salvaged/unsalvaged areas; and (2) community composition, based on a subset of 134 original species abundance matrices. We also tested the hypothesis that the impacts of salvage logging are more pronounced for saproxylic species groups than for non-saproxylic groups regarding the number of species and community composition within different types of natural disturbances.
2. Materials and Methods
2.1. Literature search
We followed guidelines for systematic literature reviews (Pullin & Stewart, 2006) to compile comparisons of species richness between salvaged and unsalvaged fire-, wind- or insect-affected forests. We screened the electronic databases Web of Science, Scopus and Google Scholar on 15 February 2016 by using the simplified search strings [salvage logging OR post$disturbance* OR salvaging] and [forest$ OR vegetation OR disturbance OR ecosystem]. From this body of literature (>2,000 articles), we retained only field-based studies after having screened the title and abstract. Modelling studies were excluded. We also added relevant papers from reference lists in published studies. We restricted studies to those providing comparisons between completely salvage logged plots and completely unsalvaged control plots according to the information given in the respective studies. This means that on salvage logged plots, more than 75% of the trees were affected by natural disturbance and then completely salvage logged without further treatment such as tree planting or legacy retention. Lower intensities of natural disturbances have been rarely targeted by scientific studies. Salvage logging operations thus resembled conventional clear-cutting. Unsalvaged control plots had to be affected by the same natural disturbance event but without any human intervention. Salvage logged plots had to be of similar size, surveyed with the same field methods during the same study period and with the same sampling effort as unsalvaged control plots.
To examine whether pseudo-replication (i.e. all plots nested within one area) might bias the results of our meta-analysis (Ramage et al., 2013), we carefully selected the studies according to their designs, and we used statistics that account for pseudo-replication (see below). The spatial arrangement of plots in all studies was checked based on method descriptions and/or original geographic coordinates. We contacted authors to provide data or to clarify their study designs where necessary (see Data sources section). Studies without true replicates (e.g. all salvaged plots nested and separated from unsalvaged control plots) were excluded from the analysis to ensure valid effect sizes (Halme et al., 2010). Studies using the same set of field plots and/or the same study area (e.g. Samcheok Forest, Korea) were identified and nested in all subsequent statistical analyses to control for pseudo-replication within study areas. We also excluded studies that sampled forests undergoing multiple types of disturbances. Salvage logging had to be conducted immediately (<12 months) after natural disturbance took place. Mean number of species and standard deviation values per sampling unit were extracted from published text and tables, or from figures using PLOT DIGITIZER 2.6.2. (www.plotdigitizer.sourceforge.net). Last, we compiled data on covariates by extracting information on the disturbance type and the time since disturbance, and the time since subsequent salvage logging. In addition, we compiled original species abundance matrices that underpinned the published papers, which allowed us to explore the effects of salvage logging on community composition.
2.2. Meta-analysis
All analyses were conducted in r 3.3.1 (www.r-project.org). Prior to statistical analysis, species were assigned to one of the following taxonomic groups and to association with dead wood (i.e. saproxylic/non-saproxylic) based on the description in the articles. These where: amphibians, ants, bats, bees and wasps, birds, carabids, epigeal lichens, epigeal mosses, epigeal spiders, epixylic lichens, epixylic mosses, harvestmen, hover flies, land snails, nocturnal moths, non-saproxylic beetles (excluding carabids), reptiles, rodents, saproxylic beetles, scuttle flies, springtails, true bugs, vascular plants and wood-inhabiting fungi. For the analysis comparing responses of saproxylic and non-saproxylic species groups, we defined saproxylic beetles, wood-inhabiting fungi, and epixylic lichens and mosses as saproxylic and all other species groups as non-saproxylic.
For comparing numbers of species between salvaged and unsalvaged naturally disturbed plots described in the published literature, we used Hedges’d, which accounts for differences in sampling effort across studies and for small sample sizes (Hedges & Olkin, 1985). Positive values of Hedges’d indicate higher numbers of species in salvage logged plots, whereas negative values indicate a loss in numbers of species attributed to salvage logging (i.e. higher numbers of species in unsalvaged naturally disturbed plots). Mean absolute effect sizes of d = 0.2 indicate a small effect, d = 0.5 a moderate effect, and d = 0.8 a large effect (Koricheva, Gurevitch, & Mengersen, 2013).
We used multi-level linear mixed-effects models, provided by the r function “rma.mv” in the “metafor” package (Viechtbauer, 2010), to test the effect of taxonomic group as a categorical predictor and year since disturbance as a numerical covariate on Hedges’d as the response variable. Hedges’d values were weighted by the corresponding sampling variance within the statistical model. Furthermore, the study site was included as a random effect in the model (i.e. moderator term) to control for unmeasured site specificities and repeated measurements (pseudo-replication) within one study site. This means that multiple data points per study were possible if studies examined multiple taxonomic groups or if studies lasted for more than 1 year. We subtracted the intercept from the effect sizes (by including “−1” in the model formula) to evaluate if observed Hedges’d differed significantly from zero (for details and model formula see Table S1).
To evaluate the effects of salvage logging on saproxylic vs. non-saproxylic groups, we fitted a second model with Hedges’d as response variable. We again included the year after natural disturbance and subsequent logging as a numerical predictor variable and study site as well as taxonomic group as random factors. Furthermore, we added the interaction of dead wood dependence (i.e. saproxylic/non-saproxylic) with natural disturbance type as predictors to test whether the effect of salvage logging on the number of species in saproxylic and non-saproxylic groups differed within different types of natural disturbances. We implemented a simultaneous inference procedure to compare saproxylic and non-saproxylic species groups within each disturbance type (Hothorn, Bretz, & Westfall, 2008). This procedure allowed us to test if responses of saproxylic and non-saproxylic taxa vary among fire-, wind- and insect-disturbed forests (for details and model formula see Table S2). Last, we conducted funnel plots by means of the function “funnel” from the “metafor” package to assess publication bias (Koricheva et al., 2013; Figure S1).
2.3. Analysis of community composition
Based on the reviewed literature, we compiled original species abundance matrices to quantify changes in community composition induced by salvage logging. Quantifying changes in community composition among large heterogeneous datasets is challenging and requires statistical methods able to deal with issues such as unbalanced sampling effort and which generate a standardized effect size that is comparable among different species groups and survey techniques. Thus, we used permutational multivariate analysis of variance using distance matrices (Legendre & Anderson, 1999), performed by means of the function “adonis” in the package “vegan” (Oksanen et al., 2016). This analysis provides a pseudo F-value, based on 999 permutations, that quantifies the deviance from the null-hypothesis, while simultaneously accounting for imbalanced study designs (McArdle & Anderson, 2001). Consequently, large values of F correspond to large changes in community composition induced by salvage logging. This F-value represents the standardized difference between communities in salvage logged and unsalvaged naturally disturbed plots within one species abundance matrix (e.g. differences in bird communities 6 years after wildfire and salvage logging in Oregon). We rigorously restricted this analysis to those abundance matrices that yielded valid pseudo F-values over the course of permutations; that is, those matrices which generated less than 99 real permutations were excluded. These restrictions resulted in a total number of 134 matrices, which supplied F-values for the analysis outlined below.
To test if salvage logging changed community composition in different taxonomic groups, we modelled pseudo F-values in linear mixed models provided by the function “lmer” in the “lme4” package assuming a Gaussian error distribution (Bolker et al., 2009). We included the taxonomic group as a categorical predictor and the year since disturbance as a numerical covariate. Furthermore, we included the study site as a random effect to control for possible differences among study sites and repeated measurements within one study site. We omitted the intercept from the model formula to determine if F-values differed significantly from zero. Thus, significant changes in community composition of a taxonomic group due to salvage logging were indicated by F-values significantly larger than zero (for details and model formula see Table S3).
As for the analysis of Hedges’d, a second model was fitted to test whether the effects of salvage logging on community composition differed between saproxylic and non-saproxylic species groups in different types of disturbances. Therefore, we included the year after disturbance and the interaction of saproxylic/non-saproxylic with disturbance type as predictors. Taxonomic group and study site were included as random factors in this model. We implemented a simultaneous inference procedure to compare saproxylic and non-saproxylic species groups within each disturbance type (for details and model formula see Table S4).
3. Results
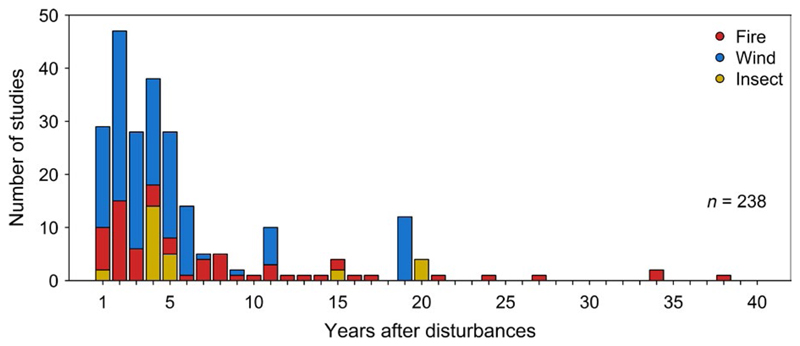
Our meta-analysis showed that the effects of salvage logging have been studied primarily for birds, vascular plants and carabids, particularly in burned forests. Studies were conducted primarily in North America and Europe, but lacking in tropical regions (Figure 1). Furthermore, there was a clear lack of studies investigating saproxylic taxa. Of the 238 compiled data points, 170 covered a period of 5 years or less after disturbance, with studies addressing the long-term effects of salvage logging being rare (Figure 2). Only one study (Hutto & Gallo, 2006) was available that provided data on the effects of salvage logging for more than 20 years after disturbances (Figure 2).
Figure 2.
Distribution of studies investigating the effects of salvage logging on biodiversity after wildfire, windstorms and insect outbreaks according to the years after disturbance.
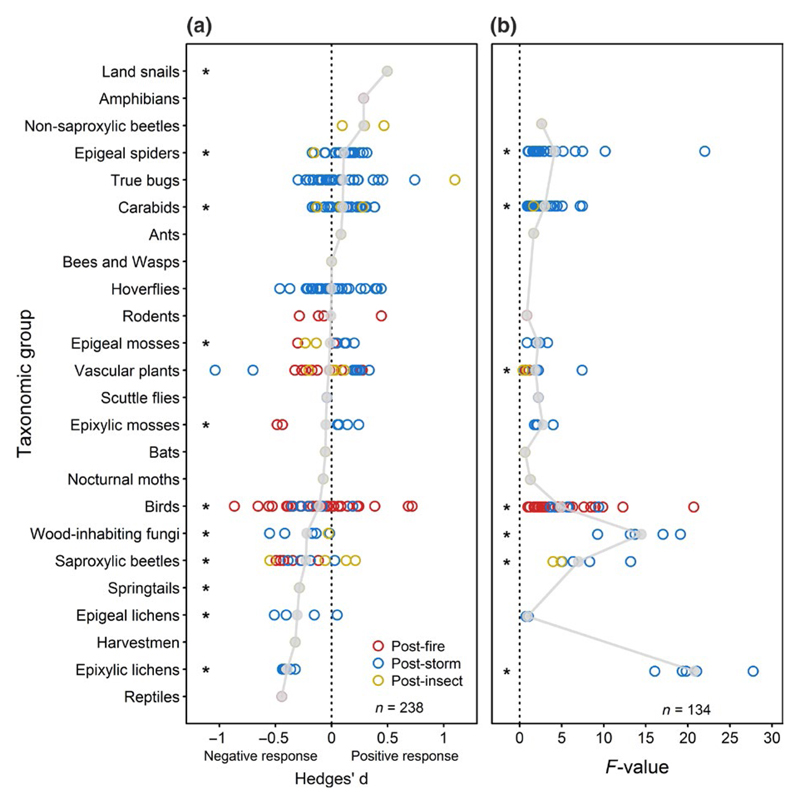
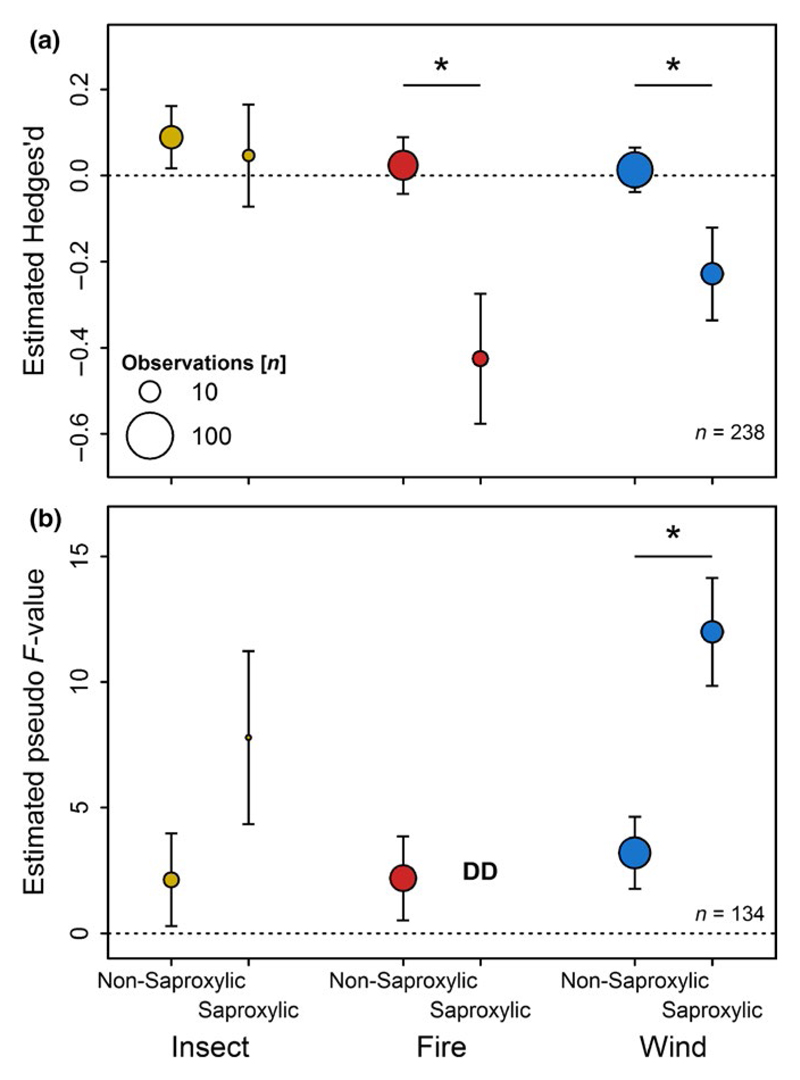
Half of the individual comparisons produced values of Hedges’d lower than zero, indicating higher numbers of species in non-salvage logged areas than salvage logged areas (Figure 3). We found significantly lower species numbers of epigeal and epixylic mosses, birds, wood-inhabiting fungi, saproxylic beetles, springtails and epixylic as well as epigeal lichens in salvage logged areas compared to non-salvage logged areas (Figure 3a). In contrast, the numbers of species of land snails, epigeal spiders and carabids were higher in salvage logged areas than in unsalvaged areas (Figure 3a). Thirteen of the 24 taxonomic groups, including vascular plants, exhibited no significant response in numbers of species to salvage logging (Figure 3a). The numbers of species of saproxylic taxa significantly decreased compared to non-saproxylic taxa in storm-affected and burned forests (Figure 4a). The negative effect of salvage logging on number of species increased with time elapsed since disturbance and subsequent salvage logging, although long-term data on salvage logging are scarce.
Figure 3.
(a) Estimated response of Hedges’ d based on 238 individual comparisons of species numbers in salvage logged and unsalvaged forests affected by natural disturbances. Higher species numbers in salvage logged areas correspond to positive Hedges’ d, whereas negative values indicate lower species numbers in salvage logged areas. (b) Pseudo F-values of permutational multivariate analysis of variance based on 134 individual species abundance matrices. Larger pseudo F-values correspond to larger changes in community composition induced by salvage logging. Asterisks indicate significant responses (see Tables S1 and S2 for statistical details). For illustrative purposes, grey dots (and the grey line joining them for emphasis) represent the mean effect size in each taxonomic group.
Figure 4.
(a) Estimated response and corresponding standard error of saproxylic and non-saproxylic taxa to salvage logging based on 238 individual comparisons (based on Hedges’ d) of numbers of species in burned, storm- and insect-affected forests. Negative estimates correspond to a decrease in numbers of species by salvage logging (Table S3). (b) Estimated response and corresponding standard error of community composition of saproxylic and non-saproxylic taxa based on pseudo F-values of permutational multivariate analysis of variance obtained from 134 individual species abundance matrices. Increasing F-values correspond to larger changes in community composition induced by salvage logging (Table S4). Note, insufficient data (DD) were available for saproxylic taxa in burned forests. Asterisks above dots indicate significant differences in the responses between saproxylic and non-saproxylic taxa within each disturbance type. Number of underlying data points is indicated by the size of the circles, with 10 and 100 size shown for reference.
Salvage logging was associated with significant changes in community composition in 7 of 17 taxonomic groups (Figure 3b). These seven groups were epigeal spiders, carabids, vascular plants, birds, wood-inhabiting fungi, saproxylic beetles and epixylic lichens (Figure 3b). Time elapsed since disturbance had no effect on the strength of logging-induced changes to community composition (Table S3). Furthermore, logging-induced changes in community composition were stronger for saproxylic taxa than for non-saproxylic taxa in storm-disturbed forests. However, data availability was scarce in insect-affected forest and lacking in burned forests (Figure 4b).
4. Discussion
Our study revealed that salvage logging can result in significant changes in species numbers and/or in altered community composition. Negative effects were particularly strong for taxa that depend on dead wood. In contrast, the numbers of species of taxa that are commonly characterized by species-rich communities in open habitats, such as carabids and epigeal spiders, responded positively to salvage logging. Despite positive effects of salvage logging on taxa associated with open habitats, strong negative effects on saproxylic groups call for substantial changes in how disturbed forests are routinely managed.
Naturally disturbed forests are characterized by large volumes of dead wood with high structural diversity (Swanson et al., 2011). In contrast, salvage logging typically reduces the amount and heterogeneity of dead wood by removing tree trunks (Keyser, Smith, & Shepperd, 2009; Priewasser, Brang, Bachofen, Bugmann, & Wohlgemuth, 2013). Not surprisingly, salvage logging reduced the numbers of species of saproxylic groups (Figures 3 and 4). However, not only a decreasing dead wood amount but likewise a logging-induced shift in dead wood quality may have additional impacts on saproxylic taxa. Salvage logging not only reduces the amount of large tree trunks but also alters characteristic conditions, such as decay stages or diameter distributions, of the remaining dead wood (Waldron et al., 2013). For instance, branches cut during post-storm logging remain on the ground but are overgrown by ground vegetation. The resulting shift in microclimatic conditions then modifies resource quality, leading to a loss of saproxylic beetles that depend on sun-exposed, dry branches (Thorn et al., 2014).
It is important to note that losses of saproxylic species can be present also within taxonomic groups that displayed no response in their overall species numbers (Figure 3a). For instance, birds (the most studied vertebrate group) were slightly negatively affected by salvage logging (Figure 3a), despite few species being directly dependent on dead wood. Nevertheless, several forest-dwelling bird species depend on snags, cavities or natural regeneration in post-disturbance forest stands. The removal of such legacies by salvage logging can cause a loss of associated bird species and consequently an overall lower number of bird species in logged areas (Hutto & Gallo, 2006; Werner, Müller, Heurich, & Thorn, 2015). Although the overall number of bird species decreased less strongly than, for instance, the number of saproxylic beetle species (Figure 3a), bird species that depend on post-disturbance habitat characteristics are often of high conservation interest. For instance, salvage logging after high severity wildfires can lead to lower site occupancies of Northern Spotted Owls (Strix occidentalis caurina) on logged than on unlogged sites in Oregon (Clark, Anthony, & Andrews, 2013).
Our study revealed that salvage logging caused significant changes in community composition for seven species groups (Figure 3b), with saproxylic species groups being affected most strongly (Figure 4b). Such alterations in community composition might reflect the establishment of open-habitat species and/or a simultaneous loss of forest specialists. For instance, salvage logging can increase the abundance of open-habitat carabid beetles (Koivula & Spence, 2006) or promote the establishment of non-forest vegetation (Stuart et al., 1993; Van Nieuwstadt, Sheil, & Kartawinata, 2001). Hence, species groups that are commonly characterized by species-rich communities in open habitats, such as carabids or epigeal spiders, can display an overall increase in numbers of species in response to salvage logging (Figure 3a). Likewise, salvage logging can cause an increase in herb- and grass-feeding moth species but a decrease in saproxylic and detritus-feeding moth species (Thorn et al., 2015). Such contrasting responses within and between species groups can mask the overall impact of salvage logging on biodiversity in coarse-scale analyses (i.e. Thom & Seidl, 2016). Numerous species of high conservation interest, such as the Red-cockaded Woodpecker (Leuconotopicus borealis), depend on dead wood in burned forests (Conner, Rudolph, & Walters, 2001). The results of our study therefore indicate that the biodiversity of saproxylic taxa could be enhanced by a modified management of naturally disturbed forests. In contrast, populations of species associated with open habitats, such as the Sharp-tailed Grouse (Tympanuchus phasianellus) in North America, may persist or even increase in the larger remaining area subject to unmodified management, that is, salvage logging (Radeloff, Mladenoff, & Boyce, 2000).
The two major incentives for salvage logging are to reduce economic losses caused by a natural disturbance and to omit mass reproduction and spread of insect pests that develop in trees killed or weakened by a preceding natural disturbance. For instance, salvage logging of storm-felled Norway spruce (Picea abies) decreased new infestations of nearby trees by the European spruce bark beetle (Ips typographus) at a landscape scale (Stadelmann, Bugmann, Meier, Wermelinger, & Bigler, 2013). Salvage logging is therefore the predominant response to natural disturbances in wood production forests, but pest control is regularly used to justify salvage logging in protected areas. For instance, the Białowieża Forest National Park on the border between Poland and Belarus, which is the last primeval lowland forest in Europe, is currently obliged to salvage logging of areas affected by I. typographus on attempt to avoid further infestations (Chylarecki & Selva, 2016). Such an approach to disturbed forests neglects that regional factors, such as summer drought, can promote outbreaks of I. typographus more strongly than local stand variables (Seidl et al., 2015). Furthermore, salvage logged timber is usually of substantially lower economic value than normally harvested timber due to a rapid colonization by wood-inhabiting fungi and to the fact that disturbances affect forests of any age, so that generalized salvage logging operations necessarily include younger stands that otherwise would not be harvested (Leverkus, Puerta-Pinero, Guzmán-Álvarez, Navarro, & Castro, 2012). Our results demonstrate that salvage logging has strong and negative effects on many taxonomic groups, particularly those associated with dead wood, and that it is thus not consistent with biodiversity conservation goals. Along with questionable economic outputs and pest reducing effects, we argue that salvage logging should be excluded from protected areas such as national parks.
The incidence of stand-replacing natural disturbances remains spatially and temporally unpredictable (Berry et al., 2015), creating inherent uncertainty about appropriate management of naturally disturbed forests. Hence, management plans need to be jointly developed with (and confirmed by) stakeholders, scientists and natural resource managers before the next disturbance occurs (Lindenmayer, Likens, & Franklin, 2010). Such management plans could, for instance, encompass an a priori identification of salvage logging exclusion zones based on ecological data (e.g. Nappi et al., 2011). Forest managers also may target the preservation of structural key attributes in naturally disturbed forests, including snags or tipped uproot plates of wind-thrown trees (Hutto, 2006). Retention of trees during green-tree harvests has become an increasingly common tool around the globe to help conserve forest biodiversity (Fedrowitz et al., 2014; Gustafsson et al., 2012; Mori & Kitagawa, 2014). To obtain some economic return while retaining dead wood-dependent taxa, we recommend a simple expansion of the green-tree retention approach to include naturally disturbed forests. Retention approaches in naturally disturbed forests could be expected to be less costly than in green-tree harvest due to the lower opportunity cost of not harvesting disturbance-killed trees.
Approximately 70% of the studies we compiled spanned less than 5 years; studies addressing the long-term effects of salvage logging are rare (Figure 2). However, dead wood, and particularly snags, are long-lasting key biological legacies, and their loss can have long-lasting effects on biodiversity (Hutto, 2006). Hence, future research should target the long-term effects of salvage logging after natural disturbances. There are also taxonomic biases in existing studies investigating the effects of salvage logging after natural disturbances. In particular, saproxylic groups such as wood-inhabiting fungi have been underrepresented in empirical studies despite their high diversity and importance for ecosystem functioning. Future research should therefore target particularly saproxylic species groups. In contrast, other groups have been relatively well studied in one disturbance type (e.g. birds in burned forests), but less in others, and studies were conducted primarily in North America, Europe and Asia, but lacking in tropical regions (Figure 1). However, different types of natural disturbances in different parts of the world can act at very different spatial scales and may require different retention approaches (Kulakowski et al., 2016). Furthermore, coniferous forests of the Northern Hemisphere—in contrast to tropical forests—are naturally prone to large-scale natural disturbances (Lindenmayer et al., 2008), whereas disturbances in tropical forests mostly have anthropogenic causes associated with long-term land-use change (e.g. fire to open space for livestock grazing and agriculture; Peres, Barlow, & Laurance, 2006). Nevertheless, natural disturbances such as windstorms affect tropical forests as well as temperate forests, and salvage logging effects on tropical forests should be targeted in future research (e.g. Lawton & Putz, 1988).
In conclusion, these data from a wide range of studies demonstrate that salvage logging has a range of effects on species numbers and community composition of various taxonomic groups, with important negative consequences for several groups, especially saproxylic ones. While current policies for enhancing biodiversity and ecosystem services, such as green-tree retention (e.g., Gustafsson et al., 2012), focus mainly on forests subjected to traditional logging operations, such policies are largely absent from naturally disturbed forests. We therefore call for an expansion of the green-tree retention approach to include naturally disturbed forests by leaving substantial amounts of dead wood on site to reduce the impact of salvage logging on biodiversity.
Supporting Information
Acknowledgements
We thank numerous contributors for clarifying their studies and three anonymous reviewers for their comments on an earlier version of this manuscript. S.T. and S.S. were funded by the German Environmental Foundation. R.S. and D.T. acknowledge support from the Austrian Science Fund (FWF, START grant Y895-B25). J.C. acknowledges support from grant P12-RNM-2705 and A.L. from Spanish MINECO (FJCI-2015-23687). D.B.L. was supported by an ARC Laureate Fellowship.
Funding information
German Environmental Foundation; Austrian Science Fund
Footnotes
Authors' Contributions
S.T. and J.M. initiated the study. S.T. analysed and interpreted the data and wrote the first draft of the paper. The authors named from S.T. to J.M. are listed alphabetically, as they contributed equally in gathering field data, providing corrections to subsequent manuscript drafts and discussing ideas.
Data Accessibility
All data are from previously published articles, see “Data sources”. Data from these articles can be made available upon reasonable request to original data owners. A list of data sources used in the study is provided in the Data Sources section.
Simon Thorn: 0000-0002-3062-3060
Alexandro B. Leverkus: 0000-0001-5452-3614
David B. Lindenmayer: 0000-0002-4766-4088
Sebastian Seibold: 0000-0002-7968-4489
Jörg Müller: 0000-0002-1409-1586
References
- Berry LE, Driscoll DA, Stein JA, Blanchard W, Banks SC, Bradstock RA, Lindenmayer DB. Identifying the location of fire refuges in wet forest ecosystems. Ecological Applications. 2015;25:2337–2348. doi: 10.1890/14-1699.1. [DOI] [PubMed] [Google Scholar]
- Blair DP, McBurney LM, Blanchard W, Banks SC, Lindenmayer DB. Disturbance gradient shows logging affects plant functional groups more than fire. Ecological Applications. 2016;26:2280–2301. doi: 10.1002/eap.1369. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Castro J, Moreno-Rueda G, Hódar J. Experimental test of postfire management in pine forests: Impact of salvage logging versus partial cutting and nonintervention on bird-species assemblages. Conservation Biology. 2010;24:810–819. doi: 10.1111/j.1523-1739.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- Choi C-Y, Lee E-J, Nam H-Y, Lee W-S, Lim J-H. Temporal changes in the breeding bird community caused by post-fire treatments after the Samcheok forest fire in Korea. Landscape and Ecological Engineering. 2014;10:203–214. [Google Scholar]
- Chylarecki P, Selva N. Ancient forest: Spare it from clearance. Nature. 2016;530:419. doi: 10.1038/530419b. [DOI] [PubMed] [Google Scholar]
- Clark DA, Anthony RG, Andrews LS. Relationship between wildfire, salvage logging, and occupancy of nesting territories by northern spotted owls. The Journal of Wildlife Management. 2013;77:672–688. [Google Scholar]
- Cobb TP, Langor DW, Spence JR. Biodiversity and multiple disturbances: Boreal forest ground beetle (Coleoptera: Carabidae) responses to wildfire, harvesting, and herbicide. Canadian Journal of Forest Research. 2007;37:1310–1323. [Google Scholar]
- Cobb TP, Morissette JL, Jacobs JM, Koivula MJ, Spence JR, Langor DW. Effects of postfire salvage logging on deadwood-associated beetles. Conservation Biology. 2011;25:94–104. doi: 10.1111/j.1523-1739.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- Conner R, Rudolph DC, Walters JR. The red-cockated woodpecker surviving in a fire-maintained ecosystem. Austin, TX: The University of Texas Press; 2001. [Google Scholar]
- Fedrowitz K, Koricheva J, Baker SC, Lindenmayer DB, Palik B, Rosenvald R, et al. Gustafsson L. Can retention forestry help conserve biodiversity? A meta-analysis. Journal of Applied Ecology. 2014;51:1669–1679. doi: 10.1111/1365-2664.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JF, Lindenmayer D, Macmahon JA, Mckee A, Perry DA, Waide R, Foster D. Threads of continuity: Ecosystem disturbances, biological legacies and ecosystem recovery. Conservation Biology in Practice. 2000;1:8–16. [Google Scholar]
- González ME, Veblen TT. Wildfire in Araucaria araucana forests and ecological considerations about salvage logging in areas recently burned. Revista Chilena de Historia Natural. 2007;80:243–253. [Google Scholar]
- Gustafsson L, Baker SC, Bauhus J, Beese WJ, Brodie A, Kouki J, et al. Franklin JF. Retention forestry to maintain multifunctional forests: A world perspective. BioScience. 2012;62:633–645. [Google Scholar]
- Halme P, Toivanen T, Honkanen M, Kotiaho JS, Mönkkönen M, Timonen J. Flawed meta-analysis of biodiversity effects of forest management. Conservation Biology. 2010;24:1154–1156. doi: 10.1111/j.1523-1739.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Hutto RL. Toward meaningful snag-management guidelines for postfire salvage logging in North American conifer forests. Conservation Biology. 2006;20:984–993. doi: 10.1111/j.1523-1739.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- Hutto RL, Gallo SM. The effects of postfire salvage logging on cavity-nesting birds. Condor. 2006;108:817–831. [Google Scholar]
- Inbar M, Wittenberg L, Tamir M. Soil erosion and forestry management after wildfire in a mediterranean woodland, Mt. Carmel, Israel. International Journal of Wildland Fire. 1997;7:285–294. [Google Scholar]
- Keyser TL, Smith FW, Shepperd WD. Short-term impact of post-fire salvage logging on regeneration, hazardous fuel accumulation, and understorey development in ponderosa pine forests of the Black Hills, SD, USA. International Journal of Wildland Fire. 2009;18:451–458. [Google Scholar]
- Koivula M, Spence JR. Effects of post-fire salvage logging on boreal mixed-wood ground beetle assemblages (Coleoptera, Carabidae) Forest Ecology and Management. 2006;236:102–112. [Google Scholar]
- Koricheva J, Gurevitch J, Mengersen K. In: Handbook of meta-analysis in ecology and evolution. Koricheva J, Gurevitch J, Mengersen K, editors. Princeton, NJ: Princeton University Press; 2013. [Google Scholar]
- Kulakowski D, Seidl R, Holeksa J, Kuuluvainen T, Nagel TA, Panayotov M, et al. Bebi P. A walk on the wild side: Disturbance dynamics and the conservation and management of European mountain forest ecosystems. Forest Ecology and Management. 2016;388:120–131. doi: 10.1016/j.foreco.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz W, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, et al. Safranyik L. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- Lain EJ, Haney A, Burris JM, Burton J. Response of vegetation and birds to severe wind disturbance and salvage logging in a southern boreal forest. Forest Ecology and Management. 2008;256:863–871. [Google Scholar]
- Lawton R, Putz FE. Natural disturbance and gap-phase regeneration in a wind-exposed tropical cloud forest. Ecology. 1988;69:764–777. [Google Scholar]
- Legendre P, Anderson MJ. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecological Monographs. 1999;69:1–24. [Google Scholar]
- Leverkus AB, Puerta-Pinero C, Guzmán-Álvarez JR, Navarro J, Castro J. Post-fire salvage logging increases restoration costs in a Mediterranean mountain ecosystem. New Forests. 2012;43:601–613. [Google Scholar]
- Lindenmayer D, Burton PJ, Franklin JF. Salvage logging and its ecological consequences. Washington: Island Press; 2008. [Google Scholar]
- Lindenmayer DB, Foster DR, Franklin JF, Hunter ML, Noss RF, Schmiegelow FA, Perry D. Salvage harvesting policies after natural disturbance. Science. 2004;303:1303. doi: 10.1126/science.1093438. [DOI] [PubMed] [Google Scholar]
- Lindenmayer DB, Likens GE, Franklin JF. Rapid responses to facilitate ecological discoveries from major disturbances. Frontiers in Ecology and the Environment. 2010;8:527–532. [Google Scholar]
- Lindenmayer DB, Noss RF. Salvage logging, ecosystem processes, and biodiversity conservation. Conservation Biology. 2006;20:946–948. doi: 10.1111/j.1523-1739.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- Lindenmayer DB, Ough K. Salvage logging in the montane ash eucalypt forests of the Central Highlands of Victoria and its potential impacts on biodiversity. Conservation Biology. 2007;20:1005–1015. doi: 10.1111/j.1523-1739.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Lindenmayer D, Thorn S, Banks S. Please do not disturb ecosystems further. Nature Ecology and Evolution. 2017;1:1–8. doi: 10.1038/s41559-016-0031. [DOI] [PubMed] [Google Scholar]
- Macdonald SE. Effects of partial post-fire salvage harvesting on vegetation communities in the boreal mixedwood forest region of northeastern Alberta, Canada. Forest Ecology and Management. 2007;239:21–31. [Google Scholar]
- McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- McIver JD, Starr L. Environmental effects of postfire logging: Literature review and annotated bibliography. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station; 2000. [Google Scholar]
- Mori AS, Kitagawa R. Retention forestry as a major paradigm for safeguarding forest biodiversity in productive landscapes: A global meta-analysis. Biological Conservation. 2014;175:65–73. [Google Scholar]
- Morimoto J, Morimoto M, Nakamura F. Initial vegetation recovery following a blowdown of a conifer plantation in monsoonal East Asia: Impacts of legacy retention, salvaging, site preparation, and weeding. Forest Ecology and Management. 2011;261:1353–1361. [Google Scholar]
- Nappi A, Drapeau P. Reproductive success of the black-backed woodpecker (Picoides arcticus) in burned boreal forests: Are burns source habitats? Biological Conservation. 2009;142:1381–1391. [Google Scholar]
- Nappi A, Stéphane D, Bujold F, Chabot M, Dumont M-C, Duval J, et al. Bergeron I. Harvesting in burned forests – Issues and orientations for ecosystem-based management. Ministère des Ressources naturelles et de la Faune, Direction de l’environnement et de la protection des forêts; Québec: 2011. [Google Scholar]
- Nikiforuk A. Empire of the beetle: How human folly and a tiny bug are killing North America’s great forests. First Edit. Vancouver, Canada: David Suzuki Foundation Series; 2011. [Google Scholar]
- Norvez O, Hébert C, Bélanger L, Hebert C, Belanger L. Impact of salvage logging on stand structure and beetle diversity in boreal balsam fir forest, 20 years after a spruce budworm outbreak. Forest Ecology and Management. 2013;302:122–132. [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. Wagner H. vegan: Community Ecology Package. 2016 Retrieved from https://cran.r-project.org/package=vegan.
- Peres CA, Barlow J, Laurance WF. Detecting anthropogenic disturbance in tropical forests. Trends in Ecology and Evolution. 2006;21:227–229. doi: 10.1016/j.tree.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Phillips ID, Cobb TP, Spence JR, Brigham RM. Salvage logging, edge effects, and carabid beetles: Connections to conservation and sustainable forest management. Environmental Entomology. 2006;35:950–957. [Google Scholar]
- Priewasser K, Brang P, Bachofen H, Bugmann H, Wohlgemuth T. Impacts of salvage-logging on the status of deadwood after windthrow in Swiss forests. European Journal of Forest Research. 2013;132:231–240. [Google Scholar]
- Pullin AS, Stewart GB. Guidelines for systematic review in conservation and environmental management. Conservation Biology. 2006;20:1647–1656. doi: 10.1111/j.1523-1739.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- Radeloff VC, Mladenoff DJ, Boyce MS. Effects of interacting disturbances on landscape patterns: Budworm defoliation and salvage logging. Ecological Applications. 2000;10:233–247. [Google Scholar]
- Ramage BS, Sheil D, Salim HMW, Fletcher C, Mustafa NZA, Luruthusamay JC, et al. Potts MD. Pseudoreplication in tropical forests and the resulting effects on biodiversity conservation. Conservation Biology. 2013;27:364–372. doi: 10.1111/cobi.12004. [DOI] [PubMed] [Google Scholar]
- Saab V, Russell RE, Dudley JG. Nest-site selection by cavity-nesting birds in relation to postfire salvage logging. Forest Ecology and Management. 2009;257:151–159. [Google Scholar]
- Schmiegelow FKA, Stepnisky DP, Stambaugh CA, Koivula M. Reconciling salvage logging of boreal forests with a natural-disturbance management model. Conservation Biology. 2006;20:971–983. doi: 10.1111/j.1523-1739.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- Seidl R, Müller J, Hothorn T, Bässler C, Heurich M, Kautz M. Small beetle, large-scale drivers: How regional and landscape factors affect outbreaks of the European spruce bark beetle. Journal of Applied Ecology. 2015;53:530–540. doi: 10.1111/1365-2664.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl R, Schelhaas M-J, Rammer W, Verkerk PJ. Increasing forest disturbances in Europe and their impact on carbon storage. Nature Climate Change. 2014;4:806–810. doi: 10.1038/nclimate2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann G, Bugmann H, Meier F, Wermelinger B, Bigler C. Effects of salvage logging and sanitation felling on bark beetle (Ips typographus L.) infestations. Forest Ecology and Management. 2013;305:273–281. [Google Scholar]
- Stokstad E. Salvage logging research continues to generate sparks. Science. 2006;311:761. doi: 10.1126/science.311.5762.761. [DOI] [PubMed] [Google Scholar]
- Stuart JD, Grifantini MC, Fox L, III, Fox L. Early successional pathways following wildfire and subsequent silvicultural treatment in Douglas-fir/hardwood forests, NW California. Forest Science. 1993;39:561–572. [Google Scholar]
- Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, et al. Swanson FJ. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Frontiers in Ecology and the Environment. 2011;9:117–125. [Google Scholar]
- Thom D, Seidl R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biological Reviews. 2016;91:760–781. doi: 10.1111/brv.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn S, Bässler C, Bernhardt-Römermann M, Cadotte M, Heibl C, Schäfer H, et al. Müller J. Changes in the dominant assembly mechanism drives species loss caused by declining resources. Ecology Letters. 2016;19:163–170. doi: 10.1111/ele.12548. [DOI] [PubMed] [Google Scholar]
- Thorn S, Bässler C, Gottschalk T, Hothorn T, Bussler H, Raffa K, Müller J. New insights into the consequences of post-windthrow salvage logging revealed by functional structure of saproxylic beetles assemblages. PLoS ONE. 2014;9:e101757. doi: 10.1371/journal.pone.0101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn S, Bässler C, Svoboda M, Müller J. Effects of natural disturbances and salvage logging on biodiversity – Lessons from the Bohemian Forest. Forest Ecology and Management. 2016;388:113–119. [Google Scholar]
- Thorn S, Hacker HH, Seibold S, Jehl H, Bässler C, Müller J. Guild-specific responses of forest Lepidoptera highlight conservation-oriented forest management – Implications from conifer-dominated forests. Forest Ecology and Management. 2015;337:41–47. [Google Scholar]
- Thorn S, Werner SAB, Wohlfahrt J, Bässler C, Seibold S, Quillfeldt P, Müller J. Response of bird assemblages to windstorm and salvage logging — Insights from analyses of functional guild and indicator species. Ecological Indicators. 2016;65:142–148. [Google Scholar]
- Van Nieuwstadt MGL, Sheil D, Kartawinata K. The ecological consequences of logging in the burned forests of East Kalimantan, Indonesia. Conservation Biology. 2001;15:1183–1186. [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- Waldron K, Ruel J, Gauthier S. Forest structural attributes after windthrow and consequences of salvage logging. Forest Ecology and Management. 2013;289:28–37. [Google Scholar]
- Werner SAB, Müller J, Heurich M, Thorn S. Natural regeneration determines wintering bird presence in wind-damaged coniferous forest stands independent of post-disturbance logging. Canadian Journal of Forest Research. 2015;45:1232–1237. [Google Scholar]
- Zmihorski M. The effect of windthrow and its management on breeding bird communities in a managed forest. Biodiversity and Conservation. 2010;19:1871–1882. [Google Scholar]
- Zmihorski M, Durska E. The effect of contrasting management types on two distinct taxonomic groups in a large-scaled windthrow. European Journal of Forest Research. 2011;130:589–600. [Google Scholar]
Data Sources
- Barnouin, T. (2005). Évaluation de L ‘ Importance Des Forêts Affectées Par La Tordeuse Des Bourgeons de L ’ Épinette, Choristoneura Fumiferana (Clem.), Dans Le Maintien de La Diversité Des Coléoptères Saproxyliques. Université Laval Quebec.
- Blair, D. P., McBurney, L. M., Blanchard, W., Banks, S. C., & Lindenmayer, D. B. (2016). Disturbance gradient shows logging affects plant functional groups more than fire. Ecological Applications, 26, 2280–2301.
- Boucher, D., Gauthier, S., Noël, J., Greene, D. F., & Bergeron, Y. (2014). Salvage logging affects early post-fire tree composition in Canadian boreal forest. Forest Ecology and Management, 325, 118–127.
- Bradbury, S. M. (2006). Response of the post-fire bryophyte community to salvage logging in boreal mixedwood forests of northeastern Alberta, Canada. Forest Ecology and Management, 234, 313–322.
- Cahall, R. E., & Hayes, J. P. (2009). Influences of postfire salvage logging on forest birds in the Eastern Cascades, Oregon, USA. Forest Ecology and Management, 257, 1119–1128.
- Castro, J., Moreno-Rueda, G., & Hódar, J. (2010). Experimental test of postfire management in pine forests: Impact of salvage logging versus partial cutting and nonintervention on bird-species assemblages. Conservation Biology, 24, 810–819.
- Choi, C.-Y. (2007). Effects of postfire logging on bird populations and communities in burned forests. Journal of the Korean Forest Society, 96, 115–123.
- Choi, C.-Y., Lee, E.-J., Nam, H.-Y., Lee, W.-S., & Lim, J.-H. (2014). Temporal changes in the breeding bird community caused by post-fire treatments after the Samcheok forest fire in Korea. Landscape and Ecological Engineering, 10, 203–214.
- Cobb, T. P., Langor, D. W., & Spence, J. R. (2007). Biodiversity and multiple disturbances: Boreal forest ground beetle (Coleoptera: Carabidae) responses to wildfire, harvesting, and herbicide. Canadian Journal of Forest Research, 37, 1310–1323.
- Cobb, T. P., Morissette, J. L., Jacobs, J. M., Koivula, M. J., Spence, J. R., & Langor, D. W. (2011). Effects of postfire salvage logging on deadwood-associated beetles. Conservation Biology, 25, 94–104.
- Donato, D. C., Fontaine, J. B., Campbell, J. L., Robinson, W. D., Kauffman, J. B., & Law, B. E. (2009). Conifer regeneration in stand-replacement portions of a large mixed-severity wildfire in the Klamath-Siskiyou Mountains. Canadian Journal of Forest Research, 39, 823–838.
- Donato, D. C., Fontaine, J. B., Kauffman, J. B., Robinson, D., & Law, B. E. (2013). Fuel mass and forest structure following stand-replacement fire and post-fire logging in a mixed-evergreen forest. International Journal of Wildland Fire, 22, 652–666.
- Duelli, P., Obrist, M. K., & Wermelinger, B. (2002). Windthrow-induced changes in faunistic biodiversity in alpine spruce forests. Forest Snow and Landscape Research, 131, 117–131.
- Durska, E. (2013). Effects of disturbances on scuttle flies (Diptera: Phoridae) in Pine Forests. Biodiversity and Conservation, 22, 1991–2021.
- Fontaine, J. B. (2007). Influences of high severity fire and postfire logging on avian and small mammal communities of the Siskiyou Mountains. Corvallis, OR: Oregon State University.
- Fontaine, J. B., Donato, D. C., Robinson, W. D., Law, B. E., & Kauffman, J. B. (2009). Bird communities following high-severity fire: Response to single and repeat fires in a mixed-evergreen forest, Oregon, USA. Forest Ecology and Management, 257, 1496–1504.
- Hutto, R. L., & Gallo, S. M. (2006). The effects of postfire salvage logging on cavity-nesting birds. Condor, 108, 817–831.
- Jeffrey, O. (2013). Effets Des Coupes de Récupération Sur Les Successions Naturelles de Coléoptères Saproxyliques Le Long D’une Chronoséquence de 15 Ans Après Feu En Forêt Boréale Commerciale. Univer.
- Jonasova, M., & Prach, K. (2008). The influence of bark beetles outbreak vs. salvage logging on ground layer vegetation in Central European mountain spruce forests. Biological Conservation, 141, 1525–1535.
- Koivula, M., & Spence, J. R. (2006). Effects of post-fire salvage logging on boreal mixed-wood ground beetle assemblages (Coleoptera, Carabidae). Forest Ecology and Management, 236, 102–112.
- Kurulok, S. E., & Macdonald, S. E. (2007). Impacts of postfire salvage logging on understory plant communities of the boreal mixedwood forest 2 and 34 years after disturbance. Canadian Journal of Forest Research, 37, 2637–2651.
- Lee, E. L. W., Hun, S., Rhim, S.-J., Lee, W.-S., & Son, S. H. (2011). Differences in bird communities in postfire silvicultural practices stands within pine forest of South Korea. Landscape and Ecological Engineering, 7, 137–143.
- Lee, E., Lee, W., & Rhim, S.-J. (2008). Characteristics of small rodent populations in post-fire silvicultural management stands within pine forest. Forest Ecology and Management, 255, 1418–1422.
- Lee, E.-J., Rhim, S., Son, S., & Lee, W. (2012). Differences in small-mammal and stand structures between unburned and burned pine stands subjected to two different post-fire silvicultural management practices. Annales Zoologici Fennici, 2450, 129–138.
- Leverkus, A. B., Lorite, J., Navarro, F. B., Sánchez-Cañete, E. P., & Castro, J. (2014). Post-fire salvage logging alters species composition and reduces cover, richness, and diversity in Mediterranean plant communities. Journal of Environmental Management, 133, 323–331.
- Liepold, K. (2004). Vergleichende Untersuchungen Zur Faunistischen Und Genetischen Diversität von Käferzönosen in Genutzten Und Ungenutzten Bergmischwäldern Des Bayerischen Waldes. TUM München.
- Mehr, M., Brandl, R., Kneib, T., & Müller, J. (2012). The effect of bark beetle infestation and salvage logging on bat activity in a national park. Biodiversity and Conservation, 21, 2775–2786.
- Norvez, O., Hébert, C., Bélanger, L., Hebert, C., & Belanger, L. (2013). Impact of salvage logging on stand structure and beetle diversity in boreal balsam fir forest, 20 years after a spruce budworm outbreak. Forest Ecology and Management, 302, 122–132.
- Park, C. (2012). Differences in characteristics of amphibian and reptile communities due to different forest environment at forest fired area in Samcheok, Gangwon Province. Seoul, Korea: Seoul National University.
- Peterson, C. J., & Leach, A. D. (2008). Salvage logging after windthrow alters microsite diversity, abundance and environment, but not vegetation. Forestry, 81, 361–376.
- Rost, J., Clavero, M., Brotons, L., & Pons, P. (2012). The effect of postfire salvage logging on bird communities in Mediterranean pine forests: The benefits for declining species. Journal of Applied Ecology, 49, 644–651.
- Rumbaitis, C. M., & Rumbaitis del Rio, C. M. (2006). Changes in understory composition following catastrophic windthrow and salvage logging in a subalpine forest ecosystem. Canadian Journal of Forest Research – Revue Canadienne De Recherche Forestiere, 36, 2943–2954.
- Thorn, S., Bässler, C., Bernhardt-Römermann, M., Cadotte, M., Heibl, C., Schäfer, H.,… Müller, J. (2016). Changes in the dominant assembly mechanism drives species loss caused by declining resources. Ecology Letters, 19, 163–170.
- Thorn, S., Bässler, C., Gottschalk, T., Hothorn, T., Bussler, H., Raffa, K., & Müller, J. (2014). New insights into the consequences of post-windthrow salvage logging revealed by functional structure of saproxylic beetles assemblages. PLoS ONE, 9, e101757.
- Thorn, S., Hacker, H. H., Seibold, S., Jehl, H., Bässler, C., & Müller, J. (2015). Guild-specific responses of forest Lepidoptera highlight conservation-oriented forest management – Implications from conifer-dominated forests. Forest Ecology and Management, 337, 41–47.
- Thorn, S., Werner, S. A. B., Wohlfahrt, J., Bässler, C., Seibold, S., Quillfeldt, P., & Müller, J. (2016). Response of bird assemblages to windstorm and salvage logging — Insights from analyses of functional guild and indicator species. Ecological Indicators, 65, 142–148.
- Waldron, K., Ruel, J.-C., Gauthier, S., De Grandpré, L., & Peterson, C. J. (2014). Effects of post-windthrow salvage logging on microsites, plant composition and regeneration ed. J. Ewald. Applied Vegetation Science, 17, 323–337.
- Wermelinger, B., Moretti, M., Duelli, P., Lachat, T., Pezzatti, G. B., & Obrist, M. K. (2017). Impact of windthrow and salvage-logging on taxonomic and functional diversity of forest arthropods. Forest Ecology and Management, 391, 9–18.
- Winter, M.-B., Ammer, C., Baier, R., Donato, D. C., Seibold, S., & Müller, J. (2015). Multitaxon alpha diversity following bark beetle disturbance: Evaluating multi-decade persistence of a diverse early-seral phase. Forest Ecology and Management, 338, 32–45.
- Zmihorski, M. (2010). The effect of windthrow and its management on breeding bird communities in a managed forest. Biodiversity and Conservation, 19, 1871–1882.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.