Abstract
Tin our investigation, we used short-time model of myocardial infarction of rats induced by high dose of isoproterenol (ISP). We investigated cardiac troponin T blood level (cTnT) and histologi-cal characteristics of rat myocardium. ISP, single, intraperitoneal dose 250 mg/kg was given to male, adult, Wistar rats (n=12). Rats were distributed depending on their body weight in subgroups: ISP I (BW 260-280g) and ISP II (BW 250-400g). Control group (n=9) was treated with intraperitoneal dose of 0,95% NaCl. Cardiac TnT was measured by electrochemiluminiscence (ECLA) sandwich immunoassay in rat serum 4 hours after ISP application. Rats’ hearts were dissected and examined by qualitative histological method (HE). Statistical significance was set at 0,05. There was significant difference in cTnT of ISP II (p=0,0001) vs. control and ISP I (p<0,05) vs. control. Significant difference was beetween ISP I and ISP II subgroups (p<0.001). The accent of histological changes of myocardium was on nuclei of cell. Cells showed acydophilic changes and nuclei disappearance as signs of coagulative necrosis development. Extensivity of histological changes were different beetween ISP I and ISP II subgroup. Used dose of ISP induced development of myocardial necrosis in rats. Suben-docardial portion of myocardium was more vulnerability than subepicardial portion. Rats of ISP II had more extensive histological changes than these in ISP I. Administered doses of ISP enabled cTnT utilization as a marker of myocardial necrosis.
Keywords: isoproterenol, cardiac troponin T, myocardial necrosis, histology
INTRODUCTION
We’re witnesses of permanent changes in diagnosing and therapy of acute myocardial infarction patients. Permanent changes are result of experimental investigations and better understanding of molecular mechanisms occurred during myocardial necrosis development. Myocardial infarction can be induced chemically and non-invasivelly in small laboratory animals like rats. Commonly used non-invasive techniques for induction of rat myocardial necrosis are those with use of cat-echolamines. For this purpose, isoproterenol-synthetic catecholamine is the most often used. It’s β-adrenergic receptors agonist and causes severe stress in myocardium resulting in infarct-like lesion. Rona and coworkers published the first results about ISP cardiotoxic effects (1). Rat myocardial changes induced by ISP are a similar to human myocardium changes during myocardial infarction (2). ISP produces a relative ischemia or hypoxia because of myocardial hyperactivity, coronary hypotension and cytosolic Ca2+ overload. There is evidence that ISP cardiotoxicity is result of catecholamine oxidation into aminochromes (3). Degree of pathomorfological changes depends on used ISP dose (4). Changes are present in subendocardial portion of myocardium, apex, left ventricle, papilar muscle and close to coronary artery. Troponins are highly sensitive and specific markers of cardiac cell damage (5). These contractile proteins are released from myocardium in proportion to the degree of tissue injury. Cardiac troponin T (cTnT) belongs to the proteins of contractile apparatus that are unique for cardiac muscle. Except in myocardial infarction diagnosing, troponins should be included in the evaluation of car-diotoxicity and cardioprotective properties of new drugs (6). There is so little information about cTnT blood level and histological finding of myocardium in ISP induced rat model of myocardial infarction. There isn’t well-standardized short time animal model of rat myocardial infarction induced by ISP for studying of: a) histological and cardiac marker changes during myocardial infarction, b) cardioprotective and c) cardiotoxic drugs investigation. In this short time rat model, we examine his-tological characteristics of cardiac muscle damage and TnT blood level in rats 4 hours after ISP administration.
MATERIAL AND METHODS
We used twelve, adult, male, Wistar rats as experimental group. They were raised and housed in air-conditioned, humidity-controlled cages. Rats had free access to water and commercial food during experimental period. Ethical Committee of our Institution approved the experiment. Rats were distributed depending on their body weight in subgroups: ISP I (BW 260-280g) and ISP II (BW 250-400g). Rats of both subgroups were treated with single intraperitoneal (i.p.) dose of ISP (250 mg/ kg BW). Control group (n=9) was treated with intrap-eritoneal dose of 0, 95% NaCl. Ketamin anesthesia (0,1 ml/100g BW) was performed in rats before of ISP administration. Isoproterenol hydrochloride was manufactured by Sigma Chemical Company, USA. Rats of ISP II died in different intervals from ISP application within 4 hours. Blood of these animals was taken by cardiac punc-tion. Rats of ISP I survived 4 hours of experimental period and blood samples were drawn from tail wein. The blood was centrifiiged for 10 minutes at 4000 r.p.m. The sera were frozen and stored at -20° until determination. The determination of cTnT was performed with Elecsys, electrochemiluminiscence (ECLIA) sandwich immu-noassay, manufactured by Roche Diagnostics. We used analyzer Elecsys 2010, Roche. Values of cTnT are given in ng/ml. The chest cavities of the rats were opened to remove the heart shortly after blood samples were taken. Rats’ hearts were dissected for histological examination. Left ventricular tissue was placed in 10% buffered formalin solution, embedded in paraffin, sectioned at 5 μιη intervals and stained with hematoxylin-eosin (HE). Histological analysis was obtained by using microscope Nikon type 400E with installated digital camera. Results of histological analysis are presented by using qualitative histological analysis. Cardiac TnT blood level data were analyzed by two-tail, unpaired Students’t-test. Significance was set at 0, 05. Results are reported as mean ± SD.
RESULTS
Control rats were treated with saline and 4 hours after that blood values of circulated cTnT were minimal.
Mean value was 0, 01 ng/ml. Four hours of experimental period after ISP aplication, rats of ISP I subgroup were survived and ISP II died in different intervals. Compared to control rats, cTnT blood level of ISP treated rats was significantly higher (p<0,0008). Mean value was 55,97 ng/ml (Figure 1).
FIGURE 1.
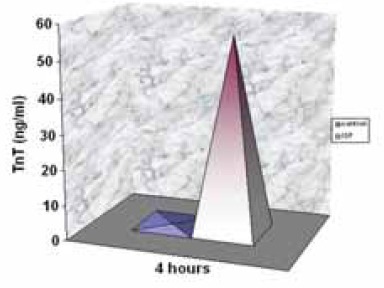
Cardiac TriT blood level in control and ISP treated rats
Significance of difference of cTnT level in ISP I vs control was p<0,05. ISP II had difference at higher level of significans p=0,0001. Mean values of cTnT in ISP I were 15,624 ± S.D.14, 44 ng/ml and for ISP II subgroup TnT was 84,80 ± 26,23 ng/ml. There was significant difference beetween ISP I and ISP II subgroups (p<0,001) (Figure 2).
FIGURE 2.
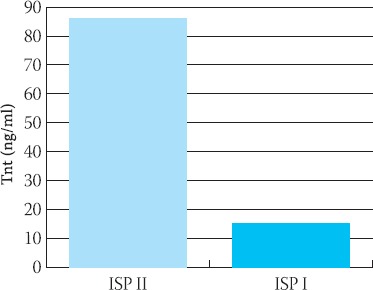
p53 positive nuclear staining in squamous cell carcinoma of lung (IH, X40).
Figure 3. (A and B) presents normal myocardial tissue of control rats. The myocardium is composed of muscle fibers. Each fiber is enclosed by a sarcolemma. The nuclei are generally located in the central portion of the fiber. A net of reticular fibers and fine collagenous fibers surround each muscle fiber. The myocardium is richly supplied with small vascular channels forming an intramural circulation.
FIGURE 3.
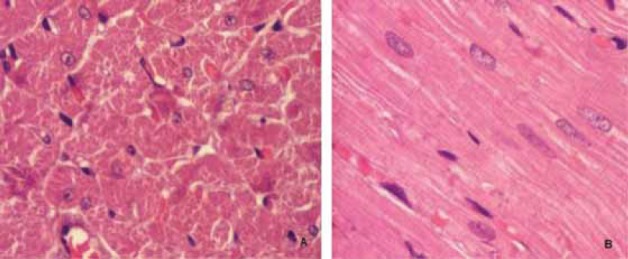
The myocardial tissue of control rat;
A) subendocardial myocardial tissue, H&E X100, B) subepicardial myocardial tissue, H&E X 400
Subendocardial myocardium of ISP I rats is presented with areas of necrotic changed myocytes. Myocites show variable degree of damage and accent of pathological changes are on nuclei. Cells are edematous, lost of the normal striation pattern and posses nuclei with inhomogeneous content (Figure 4). Deposited material of hyperacidophilic features are presented close to sar-colemma (4.B). In necrotic myocites myofibrillar lysis is complete. Deep subendocardial portion showes diffuse arranged focal necrosis of myocites. Nuclei of these areas are in phases of picnosis or lysis or completely disappearance. Blood vessels near necrotic area contain eo-sinophilic amorfic material. Subepicardial myocardium of ISP I not deviate from normal myocardial tissue of control rats.
FIGURE 4.
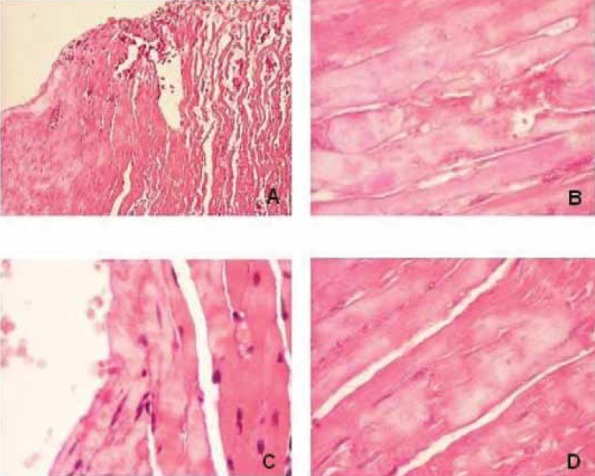
The structural changes of myocardium induced by ISP-ISP I rat (A;B) Necrotic changed cardiomyocites in subendocardial myocardium (C) Perivascular changes of myocites are more extensive (D) Deep subendocardial portion of myocardium with focal necrosis. Edematous myocites are presented with nuclei in phases of picnosis, lysis or completely disappearance (H& E X100; X 400).
Rats of ISP II died in different intervals from ISP application within 4 hours. Large blood vessels involved in sub-endocardial tissue are strong dilated (Figure 5). Histo-logical changes were more extensive compared to ISP I. Subendocardial portion is presented in form areas of massive necrosis, cellular arrangement disappeared, nuclei disappeared or in form of massive vacuola. In contrast to ISP I, rats of ISP II showes changes in subepicar-dial myocardium. Variable degree of nuclei cariolysis in subepicardial myocites is presented.
FIGURE 5.
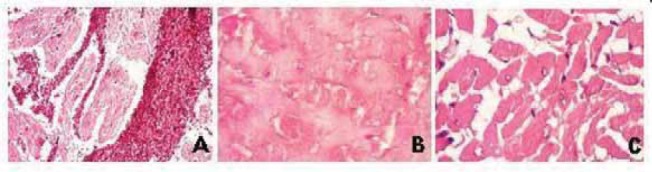
Histological characteristics of myocardium induced by ISP-ISP II rat (A) dilated and injected large blood vessel (B) massive necrosis of myocites; cellular arrangement disappeared (C)nuclei cariolysis of variable degree to completely disappearance
DISCUSSION
In this study we used rat model of acute myocardial infarction induced by ISP. Based on Rona and cowork-ers experiences, it’s known that ISP has harmful influence on heart. O’Brien and associates (7) have shown that troponins are a powerful biomarker in laboratory animals for sensitive and specific detection of cardiac injury arising from various causes. From earlier ISP studies in rats, it is known that high doses of ISP (4-80 mg/ kg) induce acute myocardial damage that has consequence in increasing of blood cTnT (8). We knew that cTnT determination with the cTnT assay, which was developed originally for human sera, was possible in the rat with the monoclonal antibodies used, because of cross-reaction between humans and rats cTnT (9). Very small amount of cTnT circulates as result of natural protein turnover. Minimal circulating cTnT values were obtained in our control rats. Detection of circulating cTnT depends on assay sensitivity. Control rats had TnT mean value 0, 01 ng/ml. Intraperitoneal, single ISP dose, administered to rats caused expectant, significant increasing cTnT. From damaged myocites, first is released free citosolic pool of cTnT. After that portion, cTnT bounded in contractile apparatus is released by proteolytic degradation. We get significant difference in cTnT blood levels between ISP II and I subgroups (p<0,001) as consequence of differrence in extensivity of histological changes. These results are in accordance to obtained results of qualitative histological analysis. Degree of myocardium pathomorfological changes depends on used ISP dose (10). Subcutaneous injection of ISP 10 mg/kg causes rat myocardial necrosis due to prolonged tachycardia. The proposed mechanism for ISP myocardial necrosis are myocardial hypoperfusion, glycogen depletion, electrolyte imbalance, lipid accumulation and free radical damage (11,12). According to Preus and coworkers, 6 hours after ISP application, cells showed acydophilic changes and nuclei disappearance as signs of coagulative necrosis development (13). There aren’t published data about using higher doses of ISP and expressed myocardial changes in first hours after application. By using ISP 250 mg/kg dose, we noted extensive myocardial lesions 4 hours from drug application. Results of histological analysis gave us clear evidence of necrogenic effect of ISP on heart. Coagulative necrosis induced by ISP, which we found in our study, is the same in case of heart damage caused by ischaemia and presented in human myocardial infarction. Massive myocardial necrosis characterizes human myocardial infarction while catecholamine model of myocardial necrosis is presented in form of focal coagulative necrosis. Joseph and Balasz described focal and multiple areas of myofibrillar necrosis after ISP application to rats (14). Our results are in concordance with their findings. Histological examination of the myocardial tissue in all ISP treated rats showed necrotic areas in the subendo-cardial layer of the left ventricle. Poorer vascularisation and oxygen supply of subendocardial portion is possible cause of more vulnerability. By qualitative histogical analysis, we noted the difference in extensivity of myo-cardial changes between ISP I and ISP II rats. Rats of ISP II had higher body weight (mean BW 325 g) than ISP I rats (mean BW 270g). ISP I subgroup have had changed subendocardial portion of myocardium, thus subepi-cardial portion didn’t show any changes. Lesions were presented in form of widespread focal necrotic areas. Cardiomyocites were most frequently without nuclei, lost of the normal myofibrilar striation pattern. There were variable degrees of damage. Rats of ISP II had expressed myocites changes in subepicardial and subendo-cardial portion of myocardium. Subendocardial alterations were more extensive than in ISP I subgroup. There were signs of massive necrosis with disappeared cellular arrangement and lost edematous and fragmented myo-cites. Large blood vessel in myocardium of ISP II group were dilated greatly and injected which present the sign of shock development and myocardial hypoperfusion. Cells’ nuclei have disappeared or presented in form of massive vacuola. Sarcoplasma of cells were fragmented. ISP II rats have had altered subepicardial portion of myocardial tissue in comparing with ISP I. Subepicardial myocites had variable degree of kariolysis. Sarcoplasma of isolated myocites showed separation and vacuoliza-tion of myofibrils. Lipolysis due to the adrenergic action of ISP is a potential factor in ISP myocardial necrosis development. The heart suplies a significant portion of its fatty acid substrates as free fatty acids derived by li-polysis from adipose tissue. Although lipid availability is important for the heart, excess level of fatty acids in cardiomyocites can be deleterious. According to Mohan, lipids mobilized from the adipose depot reach their highest level in blood one hour after ISP administration and are cleared by 4 hours (15). Lipolysis as leading critical biochemical event in induced ISP myocardial necrosis does not occur until 2 hours after ISP injection, and this time frame sugests a critical role for lipolysis. Possible mechanism of difference in extent of histological changes between ISP II and ISP I subgroup is lipolysis.
CONCLUSION
Dose of ISP (250 mg/kg) induced myocardial necrosis development in rats. Subendocardial myocardium showed more vulnerability compared to subepicardial portion. Rats of ISP II had more extensive myocardial changes than these in ISPI. Obtained results pointed at possible additive influence of lipolysis on cardiotoxic effects of ISP. Administered doses of ISP enabled cTnT utilization as a marker of myocardial necrosis. This animal model of myocardial infarction is suitable for cardiotoxic and possible cardioprotective substances investigation.
REFERENCES
- 1.Rona G. Catecholamine cardiotoxicity. J.Mol Cell Cardiol. 1985;17:123–128. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- 2.Geng B, Chang L, Pan C, Qi Y, Zhao J, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoprotere-nol. Biochem. Biophys. Res.Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 3.Remiao F, Carvalho M, Carmo H, Carvalho F, Bastos M.L. Cu2+-induced isoproterenol oxidation into isoprenochrome in adult rat calcium-tolerant cardiomyocytes. Chem. Res. Toxicol. 2002;15(6):861–869. doi: 10.1021/tx025518q. [DOI] [PubMed] [Google Scholar]
- 4.Herman E, Zhang J, Knapton A, Lipshultz E.S, Rifai N, et al. Serum cardiac troponin T as a biomarker for acute myocardial injury induced by low doses of isoproterenol in rats. Cardiovasc. Toxicol. 2006;6(3-4):211–221. doi: 10.1385/ct:6:3:211. [DOI] [PubMed] [Google Scholar]
- 5.Babuin L, Jaffe A.S. Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ. 2005;173(10):119–140. doi: 10.1503/cmaj.050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace K.B, Hausner E, Herman E, Holt G.D, Macgregor J.T, et al. Serum troponins as biomarkers of drugs-induced cardiac toxicity. Toxicol. Pathol. 2004;32:106–121. doi: 10.1080/01926230490261302. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien P.J, Smith D.E.C, Knechtel T.J, Marchak M.A. Pruim-boom-Brees i Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab. Anim. 2006;40:153–171. doi: 10.1258/002367706776319042. [DOI] [PubMed] [Google Scholar]
- 8.Herman E.H, Zhang J, Knapton A, Rifat N, Sistare F, et al. The utility of monitoring cardiac troponin T to detect cardiac injury induced by low doses of isoproterenol in rats. FDA SCIENCE The critical path from concept to consumer, 11th Annual FDA Science Forum. 2005 [Google Scholar]
- 9.Bertinchant J.P, Robert E, Polge A, Marty-Double C, et al. Comparison of the diagnostic value of cardiac troponin I and T determination for detecting early myocardial damage and the relationship with histological findings after isoprenaline-induced cardiac injury in rats. Clin. Chim. Acta. 2000;298:13–28. doi: 10.1016/s0009-8981(00)00223-0. [DOI] [PubMed] [Google Scholar]
- 10.Acikel M, Buyukokuroglu M.E, Erdogan F, Aksoy H, Bozkurt E, Senocak H. Protective effects of dantrolene against myocardial injury induced by isoproterenol in rats: biochemical and histo-logical findings. Int. J. Cardiol. 2005;98:389–394. doi: 10.1016/j.ijcard.2003.10.054. [DOI] [PubMed] [Google Scholar]
- 11.Acikel M, Buyukokuroglu M.E, Aksoy H, Erdogan F, Erol M.K. Protective effects of melatonin against myocardial injury induced by isoproterenol in rats. J.Pineal. Res. 2003;35:75–79. doi: 10.1034/j.1600-079x.2003.00056.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajadurai M, Prince P.S.M. Comparative effects of Aegle marme-los extract and α-tocopherol on serum lipids, lipid peroxide and cardiac enzyme levels in rats with isoproterenol-induced myocar-dial infarction. Singapore Med. J. 2005;46(2):78–81. [PubMed] [Google Scholar]
- 13.Preus M, Bhargava A.S, Khater Abd El R, Gunzel P. Diagnostic value of serum creatine kinase and lactate dehydrogenase isoen-zyme determinations for monitoring early cardiac damage in rats. Toxicol. Lett. 1988;42:225–233. doi: 10.1016/0378-4274(88)90081-1. [DOI] [PubMed] [Google Scholar]
- 14.Joseph X, Balazs T. Protection against isoproterenol-induced myocardial necrosis in rats by 6-mercaptopurine and 6-thiofua-nine or by irradiation. Res. Com. Chem. Path. Pharmacol. 1989;63(3):385–394. [PubMed] [Google Scholar]
- 15.Mohan P, Bloom S. Lipolysis is an important determinant of isoproterenol-induced myocardial necrosis. Cardiovasc. Pathol. 1999;8(5):255–261. doi: 10.1016/s1054-8807(99)00017-4. [DOI] [PubMed] [Google Scholar]


