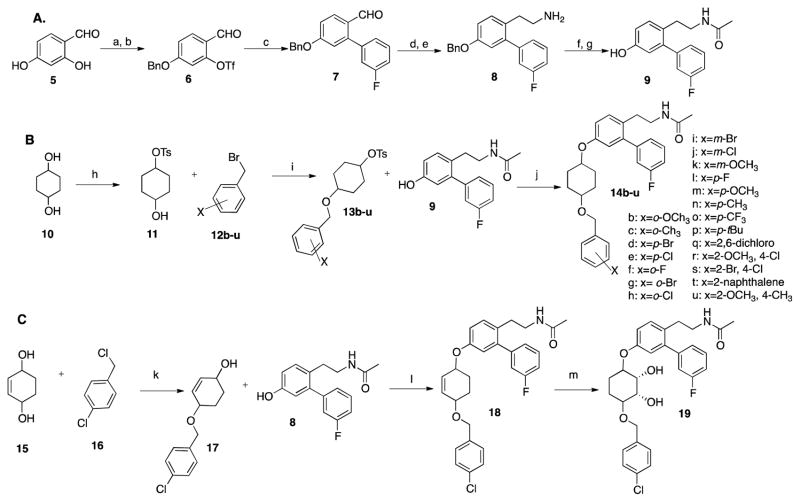
Scheme 1.
A). Synthesis of phenol 9. Reaction conditions to make phenol 9: a. BnBr, NaHCO3, CH3CN, 75%; b.(CF3CO)2O, Et3N, DCM, 60%; c.3-fluoro-phenylboronic acid, Pd(PPh3)4, K2CO3, DMF, 90%; d. CH3NO2, NH4OAc, 98%; e. LiAlH4, THF, 30 min, f. Ac2O, Et3N, 72% over 2 steps, g. H2, Pd/C, MeOH, 90%. B). Synthesis of Noviomimetics 14b–u: h. TsCl, Pyridine, 0 °C-rt, on CHCl3; i. NaH, Acetonitrile, rt, 16hr; j. K2CO3, DMF, 80°C, 2d, 55–80%; b: x = o-OCH3; c: x = o-CH3; d: x = p-Br; e: x = p-Cl; f: x = o-F; g: x = o-Br; h: x = o-Cl; i: x = m-Br; j: x = m-Cl; k: x = m-OCH3, l: x = p-F; m: x = p-OCH3; n: x = p-CH3; o: x = p-CF3; p: x = p-tBu; q: x = 2, 6-dichloro; r: x = 2-OCH3, 4-Cl; s: x = 2-Br, 4-Cl; t: x = 2-naphthalene; u: x = 2-OCH3, 4-CH3. C). Synthesis of compounds 18 and 19: k. NaH, DMF, rt, on, 60%; l. PPh3, DIAD, THF, rt, 3h, 58%; m. OsO4, NMO, THF/H2O, on, 83%.