Abstract
Background
Papillary thyroid carcinoma (PTC) has excellent survival, yet recurrence remains a challenge. We sought to determine the proportion of re-operations performed for persistent, rather than truly recurrent disease.
Methods
We conducted a retrospective review of a prospectively maintained database. Patients with PTC that had re-operation for disease from 2000–2016 were included. We defined recurrence as disease that developed after a patient had an undetectable thyroglobulin and negative ultrasound within one year of surgery.
Results
A total of 69 patients underwent 92 re-operations. On initial pathology: mean tumor size was 2.6cm; 50.7% were multifocal; and 42% had extra-thyroidal extension. Half (46%) of the patients underwent a central/lateral neck dissection at initial surgery and 76.8% were treated with post-operative radioactive iodine. The median time to first re-operation was 21 months (range, 1–292), and 41.8% occurred within 1 year. Only three operations met criteria for true “recurrence”, while 71 operations were categorized as persistence.
Conclusion
Many re-operations for PTC are for management of persistent disease. Over half of the patients required re-operation within the first two years, which strongly suggests that improvements in the pre-operative assessment and adequacy of initial surgery need to be made to improve the care of patients with thyroid cancer.
Keywords: Papillary thyroid cancer, reoperation, recurrence, persistence, thyroglobulin
Introduction
Papillary thyroid carcinoma (PTC) has excellent survival, however, recurrence remains a major concern with up to 20% of patients developing recurrent disease at some point during their lifetime(1). The average time to recurrence has been reported in the literature anywhere from 6 months to decades later (2–4). The following factors are associated with a higher risk of recurrence: lymph node metastasis, histologic variant, tumor size, extra-thyroidal extension, extra-nodal extension, male sex, and age above 45 years old at time of diagnosis (5, 6). The American Thyroid Association (ATA) risk stratification for well-differentiated thyroid cancer categorizes patients into low, intermediate, and high risk groups based on these factors(7). Patients that fall into ATA intermediate and high risk groups have been shown to have higher rates of recurrence when applied to the clinical setting, with rates the highest in the high risk category (8).
Fortunately, various tools and treatment recommendations are available to help detect PTC recurrence. Thyroglobulin (Tg) as a tumor marker for recurrent disease was first described early on by LoGerfo and colleagues (9). A Swedish group further applied this tool to show increases in Tg levels corresponded to disease and recommended its use as an early marker to detect recurrence (10). Radiographic studies, specifically ultrasound, in conjunction with Tg, have emerged as standard surveillance tools for detection of recurrence in patients with PTC.
The definition and timeline of recurrence and persistence for PTC is not clearly defined in the literature. The most recent ATA guidelines define “disease free status” as the following: 1-no clinical evidence of tumor, 2-no evidence of tumor by RAI imaging and/or neck ultrasound, 3-unstimulated Tg <0.2 ng/mL or stimulated Tg <1 ng/mL in the absence of interfering antibodies (7). Given this definition of disease free status, it is unclear how many patients that undergo a re-operation for “recurrent” disease truly had a disease free state previously. Understanding this risk is essential to help stratify patients post-operatively and allow us to tailor their follow up plan based on their risk of recurrence. This information can also be used to highlight the importance of the adequacy of initial surgical management to optimize outcomes for thyroid cancer patients.
The aim of this study was to characterize patients that underwent re-operative surgery for PTC and to examine what percentage of re-operations are truly for “recurrent” disease versus management of persistent disease. We hypothesized that patients that had earlier re-operations likely had persistent disease, and Tg and ultrasound abnormalities would support this classification.
Methods
We conducted a retrospective review of a prospectively maintained thyroid surgical database. All patients that had re-operative surgery for PTC from 2000–2016 who had the entire thyroid previously removed, either through a one-stage or two-stage procedure (i.e. completion thyroidectomy) were included. Demographic variables, pre-operative and post-operative imaging and laboratory results, and pathology results from both the initial surgery as well as re-operative surgeries, were collected and analyzed. We specifically focused on Tg levels and neck ultrasound exams. We recorded both stimulated and unstimulated Tg values at both pre-operative and post-operative time points when available. For those who underwent post-operative radioactive iodine treatment, we focused on post-treatment values.
We classified disease as a true “recurrence” if a patient had an undetectable Tg in the absence of Tg antibodies and a negative neck ultrasound within one year of the previous surgery. We defined persistent disease as having a positive Tg level, an abnormal ultrasound, or persisting elevated Tg antibodies. Specifically, an undetectable Tg was defined as <0.2 ng/mL for unstimulated and <1.0 ng/mL for stimulated (7). An ultrasound was defined as abnormal if it included any of the following phrases: abnormal tissue in thyroid bed, abnormal or suspicious lymph nodes in the central or lateral neck, increase in growth of mass or lymph nodes, or invasion of surrounding structures. Time to recurrence was measured from the date of the most recent neck operation.
Descriptive statistics were reported as a mean with a standard deviation for continuous variables or as a frequency with a percent for nominal variables. Kaplan-Meier and cox regression models were used to evaluate time to re-operation. All analyses were performed using SPSS 23 (IBM, Chicago, IL). The Institutional Review Board of the University of Wisconsin, Madison approved this study and patient information was protected in accordance with the Health Insurance Portability and Accountability Act.
Results
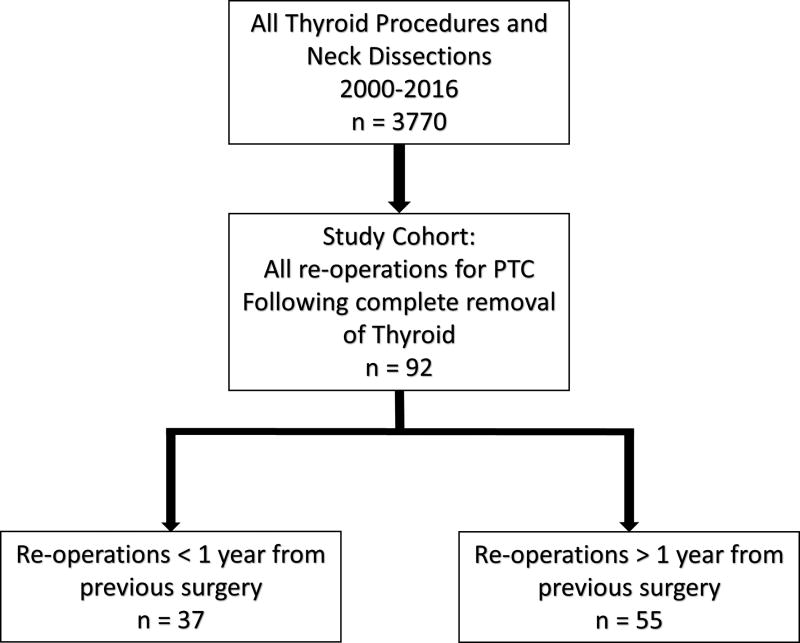
Our cohort included 69 patients undergoing a total of 92 re-operations for PTC, with 19 patients having more than one re-operation for recurrent or persistent disease (Figure 1). When evaluating the initial surgery, 46% (n=32) of patients had some sort of neck dissection at the same time as their initial thyroidectomy (Table 1). Most (78%) initial operations were performed at referring facilities, while 22% of initial operations were performed at our institution.
Figure 1.
Study cohort
Table 1.
Central and Lateral neck dissections performed at initial operation
| N (=32) | Average nodes positive (SD) |
Average nodes removed (SD) |
Average LN ratio (SD) |
|
|---|---|---|---|---|
| CND | 12 | 5.4 (±4.9) | 8.3 (±7.9) | 0.75 (±0.25) |
| MRND | 3 | 10.3 (±5.8) | 15.7 (±8.5) | 0.72 (±0.28) |
| CND & MRND | 17 | 15.5 (±8.6) | 34.8 (±21.8) | 0.50 (±0.18) |
CND=central neck dissection, MRND=modified radical neck dissection
Demographics and Characteristics of Initial Surgery
The majority of patients were female (69%) and the mean age was 42 years, with 55% being less than 45 years old (Table 2). Initial tumor pathology was papillary thyroid cancer in all patients and included variants such as follicular (9%), tall cell (7%), and sclerosing (3%). On pathology review, the average tumor size was 2.7cm, 50% had multi-focal disease, 35% had extra-capsular invasions, 42% had extra-thyroidal extension, and 30% reported lympho-vascular invasion. When categorized using the ATA risk stratification, 14.5% were in the high-risk group (n=10), 56.5% in the intermediate risk group (n=39), 15.9% in the low risk group (n=11), and the remaining 13.1% (n=9) were unable to be classified. Of the 69 patients, 77% of them went on to receive radioactive iodine treatment after the initial surgery (Table 2, 3).
Table 2.
Patient Demographics and characteristics of initial operation
| Variables | All patients (n=69) | % |
|---|---|---|
| SEX, n (%) | ||
| Male | 21 | 30.4 |
| Female | 48 | 69.5 |
| AGE, n(%) | ||
| Mean age, years (SD) | 42.4 (±14.6) | |
| <45 | 38 | 55.0 |
| >45 | 31 | 44.9 |
| Family History PTC | 19 | 27.5 |
| History of Radiation | 2 | 2.9 |
| Initial Surgery Location | ||
| Referring facility | 54 | 78.2 |
| University of Wisconsin -Madison | 15 | 21.7 |
| PRIMARY TUMOR | ||
| Histologic subtype | ||
| Classical PTC | 56 | 81.2 |
| Follicular variant | 6 | 8.6 |
| Tall cell | 5 | 7.2 |
| Sclerosing | 2 | 3.0 |
| Mean size, cm (SD) | 2.66 (±1.55) | |
| Multi-focality | 35 | 50.7 |
| Capsular invasion | 24 | 34.7 |
| Extra-thyroidal extension | 29 | 42.0 |
| Lympho-vascular invasion | 21 | 30.4 |
| ATA Risk Stratification | ||
| Low | 11 | 15.9 |
| Intermediate | 39 | 56.5 |
| High | 10 | 14.4 |
| Post-operative RAI | 53 | 76.8 |
SD-Standard deviation, PTC-Papillary thyroid cancer, ATA-American Thyroid Association, RAI-Radioactive iodine
Table 3.
TNM Staging - Initial 69 Operations
| All patients (n=69) | % | |
|---|---|---|
| TNM Classification | ||
| T1 | 11 | 15.9 |
| T2 | 5 | 7.2 |
| T3 | 43 | 62.3 |
| T4 | 1 | 1.4 |
| Nx | 20 | 29.0 |
| N0 | 0 | 0.0 |
| N1a | 20 | 29.0 |
| N1b | 20 | 29.0 |
| M0 | 60 | 87.0 |
| M1 | 0 | 0.0 |
| Stage | ||
| Stage I | 36 | 52.2 |
| Stage II | 1 | 1.5 |
| Stage III | 13 | 18.8 |
| Stage IVA | 9 | 13.0 |
| Stage IVC | 1 | 1.5 |
| Unable to classify | 9 | 13.0 |
Time to Re-operation
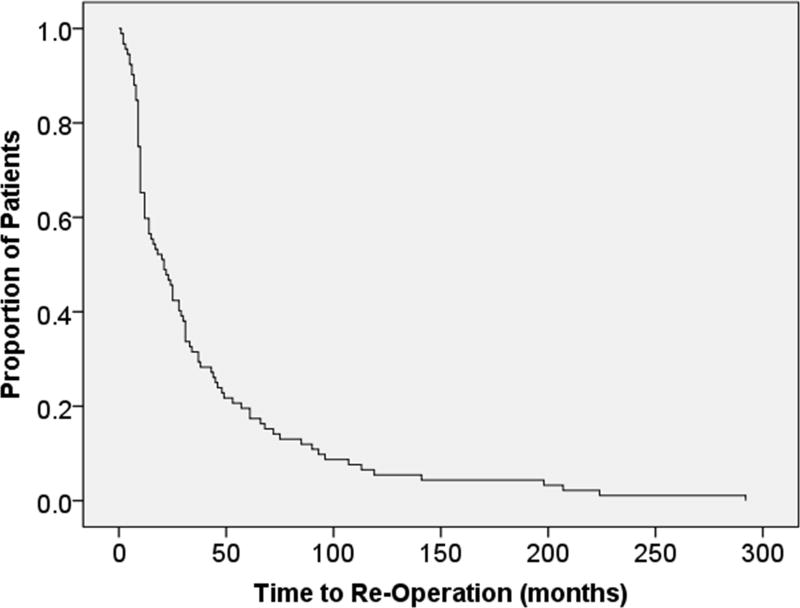
We examined time from initial operation to first re-operation in all 69 patients. The median time to re-operation was 21 months, with a range of one month to 292 months (Figure 2). Most re-operations (77.6%) occurred within the first 5 years, 52.6% were in the first two years, and 41.8% were within 1 year from initial surgery.
Figure 2.
Time to re-operation
This curve depicts the time in months to re-operation, where over 50% of the cohort underwent re-operation by 24 months.
Thyroglobulin and Ultrasound Results
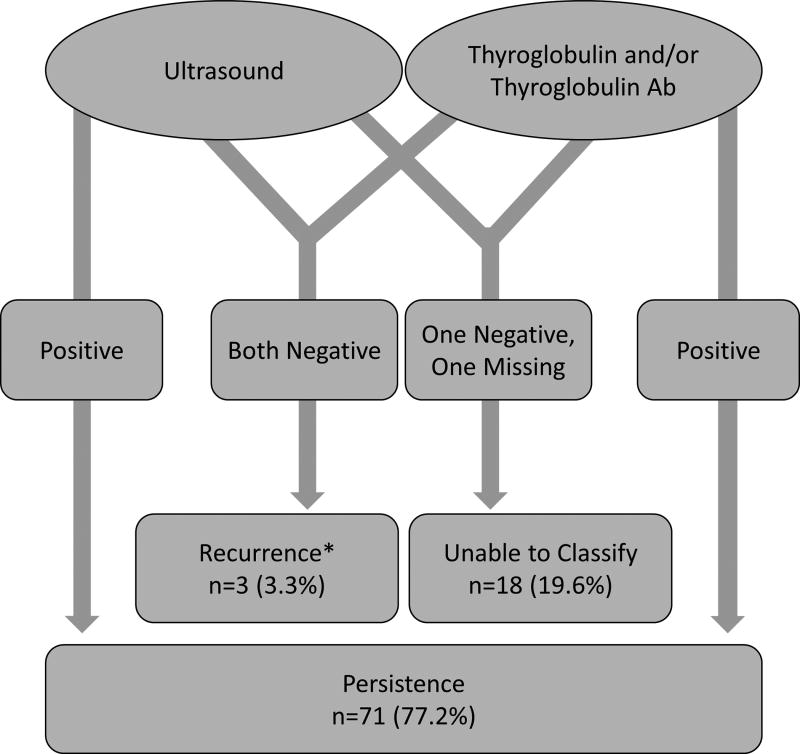
We classified patients as having persistent or recurrent disease based on ultrasound, Tg, and Tg antibodies within 1 year of the initial operation (Figure 3). We classified 71 re-operations (77.2%) as persistent disease. Of re-operations for persistent disease, 80% had an abnormal ultrasound, 70% had a positive Tg level, and 13% had persistently elevated Tg antibodies within one year of surgery. Only 3 re-operations were performed for truly recurrent disease based on a strict definition of a negative Tg, no Tg antibodies, and a negative ultrasound prior to re-operation. These patients were re-operated on at time points of 14 months, 37 months, and 93 months after their initial surgery. The mean time to recurrence in these patients was 48 months in comparison to the entire cohort which was only 21 months. Eighteen re-operations (19.6%) had incomplete follow up data and therefore could not be accurately categorized as either persistent or recurrent disease. 19 patients had multiple re-operations. A subset analysis was done including first re-operations only and outcomes were comparable to the results above. All 3 recurrences occurred in patients at the first reoperation (n=3, 4.3%). The additional 23 reoperations on these 19 patients all had either a positive Tg and/or positive ultrasound within one year after previous surgery. None met our definition of a true recurrence.
Figure 3.
Ultrasound and Thyroglobulin results for first 12 months post-operatively
Re-operations were classified as persistent or recurrent disease based on ultrasound, Tg, and Tg antibody results for the first 12 months after the previous surgery.
*Recurrences later went on to have an elevated Tg and/or abnormal ultrasound after one year.
The median follow up time of this cohort was 24 months (1–158), where 19 patients were lost to follow up and 6 patients passed away, all due to complications from metastatic PTC.
Patients with “recurrent” disease
We classified 3 patients as having true recurrence based on an undetectable Tg level and a negative neck ultrasound within one year after their initial surgery. Patient 1 underwent total thyroidectomy and left central neck dissection with pathology reporting 3 of 9 nodes positive for classical PTC. Postoperatively, they had an undetectable Tg and negative ultrasound at 7 months. At 13 months, a repeat ultrasound showed left lateral neck adenopathy with fine needle aspiration confirming nodal disease. A left lateral neck dissection was performed and pathology reported 2 of 33 nodes positive for PTC. Patient 2 underwent total thyroidectomy with left central neck dissection with 2 of 2 nodes positive for classical PTC. Tg was undetectable and ultrasound was negative at 12 months postoperatively. Tg first became elevated to 0.4 ng/mL 79 months later, where ultrasound at 84 months identified a left central neck mass. Repeat left central neck dissection was performed 93 months after initial surgery and pathology reported 5 of 5 nodes positive for PTC. Patient 3 had sclerosing variant of PTC on initial pathology and had an undetectable Tg and negative ultrasound at 6 months postoperatively. Tg was next checked 36 months later and elevated to 80 ng/mL with an ultrasound showing a right central neck mass. A right central neck dissection was performed removing one large node that was positive for sclerosing variant of PTC. This patient subsequently developed metastatic disease and expired.
Discussion
Currently all re-operations after an initial total thyroidectomy for thyroid cancer are labeled as a recurrence. We questioned whether these re-operations were appropriately labeled as a recurrence and if most re-operations are truly for management of persistence disease. While we recognize that recurrence is a major problem for patients with thyroid cancer, we were interested in determining how often a re-operation is needed in patients who achieve an optimal outcome after their initial treatment (both a negative US and undetectable Tg level). We examined all patients that had re-operative surgery for PTC and we found that most patients required their re-operation in less than 24 months and almost half within the first year. In reviewing these cases, we could only identify three cases that represented a true disease recurrence after a previous time in which their neck US was negative and their Tg was undetectable. What we found was that almost all re-operations for thyroid cancer had evidence of persistent disease at their first follow up after surgery and that these re-operations were really for ongoing management of persistent disease. This highlights that the problem of recurrent disease in thyroid cancer which can be best addressed by optimizing our initial evaluation and management of these patients.
Stimulated and unstimulated Tg levels have been available for the last several decades as markers of disease surveillance, and are now utilized to determine post-operative treatment, specifically the need for radioactive iodine or the need for repeat surgical intervention (11). Several studies have shown that undetectable post-operative Tg levels correspond with a low incidence of recurrence, while elevated Tg levels are associated with recurrence (12–15). Rosario and colleagues demonstrated that Tg levels often detected recurrent PTC, even prior to structurally detectable disease on ultrasound (16). One study evaluating the risk of recurrent PTC demonstrated an increase in Tg more than 2 fold was seen in 80% of patients with recurrence and only 13% of patients without recurrent PTC (17). In our cohort, the majority of patients had persistently elevated Tg levels after surgery suggesting that disease or at least thyroid tissue was still present after their initial treatment. While a persistently elevated Tg level after surgery could represent a normal thyroid remnant, those patients are not necessarily captured in our dataset as all patients in our study subsequently had a re-operation with pathologically proven disease.
We considered persistently elevated Tg antibodies abnormal and interpreted them as a potential marker of persistent disease. While the presence of Tg antibodies does not necessarily equate with disease recurrence, we felt that persistently elevated Tg levels could be masking persistent disease and we felt it was safest to assume the worst case for our analysis, so that we could have the cleanest definition for recurrence. The significance of a persistently elevated Tg antibody after surgery is unclear. Xu and colleagues examined trends in Tg antibody levels after total thyroidectomy for thyroid cancer and found that Tg Ab levels resolved in the majority of patients (25/35) but took a median of 11 months post-surgery to resolve. In their cohort of patients with persistently elevated Tg Ab, there was only one patient who had to undergo a re-operation for proven disease (18). However, another group looked at recurrences in PTC and changes in serum Tg antibodies and stratified into categories: persistently high, rising Tg antibodies, persistently medium, decreasing Tg antibodies, and decreasing to negative. Patients with persistently high, increasing, and persistently medium Tg antibodies had more disease (P<0.001). In addition, the trend of Tg antibody changes was a strong predictor of PTC disease on multivariable analysis (19). While the presence of Tg Ab after surgery does not necessarily correlate with persistent disease, it is expected that they should resolve or decrease over time. If they do not, then they likely represent persistent disease.
Ultrasound is recommended as a tool for surveillance of postoperative disease in PTC (7). In our study, 80% of re-operations had an abnormal ultrasound within one year from their previous surgery. Miah and colleagues looked at the utility of ultrasound post-thyroidectomy and demonstrated that lymph node mapping can detect recurrent PTC when Tg levels are undetectable (20). Other studies supported the usefulness of neck ultrasound in the early diagnosis of recurrence, where one study reported a 31.5 times greater chance of recurrent disease if there were detectable abnormalities (i.e. masses, lymphadenopathy) by ultrasound in the thyroid bed (12, 17). Ultrasound has been advocated in the pre-operative evaluation of patients prior to their initial surgery, but adherence with this recommendation has been variable and there is significant variation in the quality of pre-operative neck ultrasound to evaluate for lymph node involvement (21). Lacking or inadequate preoperative imaging is likely a contributing factor to persistent disease recognized in the early post-operative period. Much of the disease identified post-operatively at 6 months or 1 year after surgery was likely present at the initial surgery, but simply not recognized.
When examining our cohort, it is important to consider the variables that contribute to the risk of recurrence. Almost half of our patients had some sort of lymph node dissection at their initial surgery. Patients with nodal disease at their initial surgery have higher incidences of recurrence than those patients without lymph node involvement (5). In addition, Poehls and colleagues reported that positive nodal disease on pre-operative ultrasound corresponded with higher rates of repeat surgery compared to those with a negative pre-operative ultrasound (22). The risk of recurrence in patients with lymph node disease is also related to the number and ratio of lymph nodes involved. If patients have a large number of nodes involved or have a significantly higher lymph node ratio, (number of positive nodes/number of nodes removed) then they are at significantly higher risk of recurrence (23, 24). While nodal recurrence can be a reflection of the initial extent of disease, it may also reflect the adequacy of the initial surgical management. Besides lymph node involvement at initial surgery, our cohort had other risk factors for recurrence. On initial pathology, extra-thyroidal extension and lympho-vascular invasion were common and the clear majority were classified as intermediate or high risk for recurrence per the ATA risk stratification. These variables have been show to increase the risk of recurrence which is applicable to our cohort (5, 6).
The most alarming finding from our data was the short interval between the initial operation and the re-operation, and the fact that most patients never achieved a disease-free state, suggesting these re-operations were not for true recurrence, but instead for persistent disease. Specifically, in patients that required multiple reoperations, none achieved disease free status after any reoperation, suggesting that it is difficult to achieve disease free status the second or third or fourth time around. Though there are some patient and disease risk factors that surgeons and providers cannot change (advanced disease at presentation, tumor biology, etc.) – there are other areas that we can influence outcomes. Specifically, the importance of a thorough pre-operative lymph node evaluation, the quality of the initial surgery, and the appropriate post-operative treatment and follow-up. Onkendi and colleagues evaluated a cohort of patients that underwent re-operation for recurrent PTC and reported that improved surgical technique or pre-operative localization might have decreased recurrence risk in 15–20% of their cohort. Inadequate pre-operative ultrasound in 5%, nodal recurrence in the operative field in 10%, and a combination of the two in 3% were cited as reasons for relapse (25). We must ensure all lymph node compartments are adequately assessed on imaging and any abnormal appearing nodes interrogated. Central and lateral neck dissections should not only include only the grossly positive nodes but a healthy margin of normal appearing lymph tissue to ensure microscopic disease is removed. Short-term markers such as Tg levels and lymph node ratios should be tracked by surgeons regularly in the postoperative period and used as tools to guide quality improvement measures. It is important that we strive for continuous improvement in our initial surgical management, in order to reduce the morbidity from reoperative surgery in these patients.
There are several limitations of this study. This was a retrospective study and we were limited by the data that was available in the medical record for review. This was a particular problem when trying to evaluate the cause of the persistent disease. Since most of the initial surgeries were done outside our institution, it was difficult to determine the quality of pre-operative staging and operative management. Since a relatively small number of patients were considered to be disease free after their initial surgery this prevented us from doing a comparative analyses between those patients with persistent or recurrent disease. In addition, our conclusions can only be applied to patients with PTC that underwent reoperation, as our cohort did not include PTC patients who did not require a reoperation. There may also be a selection bias as many of these patients were referred to a tertiary care center and thus the severity of their disease may be higher than the average thyroid cancer patient. Additionally, there was a small subset of patients that did not have all laboratory and radiography results documented or available at all examined time points limiting our ability to classify all patients as having persistent or recurrent disease based on the results that were available. While the post-operative surveillance schedule is standardized at our institution, a large part of our cohort was referred to our institution and there is likely significant variability in both the preoperative and post-operative follow up of patients.
Our examination of re-operations for PTC showed a large number of patients who underwent early re-operation due to postoperative findings on ultrasound or elevated Tg levels demonstrating persistent disease. Many factors may contribute to this end result including: the quality and extent of pre-operative evaluation and the initial operation, and post-operative treatment with radioactive iodine. Improvements in these areas are critical to improving the care of patients with thyroid cancer, decreasing their lifetime risk of recurrence, and avoiding unnecessary or unindicated RAI treatment, and preventing avoidable re-operations.
Acknowledgments
Support for this research included the NCI-NIH award number R01CA176911. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Information: Nothing to Disclose
Presented as a podium presentation at the 38th annual meeting for the American Association of Endocrine Surgeons, April 2–4, 2017 in Orlando, FL
References
- 1.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 2.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154(6):1436–46. doi: 10.1016/j.surg.2013.07.008. discussion 46-7. [DOI] [PubMed] [Google Scholar]
- 3.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, et al. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab. 2013;98(2):636–42. doi: 10.1210/jc.2012-3401. [DOI] [PubMed] [Google Scholar]
- 4.Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O, Toubeau M, et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011;96(5):1352–9. doi: 10.1210/jc.2010-2708. [DOI] [PubMed] [Google Scholar]
- 5.Liu FH, Kuo SF, Hsueh C, Chao TC, Lin JD. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol. 2015;112(2):149–54. doi: 10.1002/jso.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito Y, Kudo T, Kobayashi K, Miya A, Ichihara K, Miyauchi A. Prognostic factors for recurrence of papillary thyroid carcinoma in the lymph nodes, lung, and bone: analysis of 5,768 patients with average 10-year follow-up. World J Surg. 2012;36(6):1274–8. doi: 10.1007/s00268-012-1423-5. [DOI] [PubMed] [Google Scholar]
- 7.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo JY, Stang MT. Current Guidelines for Postoperative Treatment and Follow-Up of Well-Differentiated Thyroid Cancer. Surg Oncol Clin N Am. 2016;25(1):41–59. doi: 10.1016/j.soc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Gerfo PL, Stillman T, Colacchio D, Feind C. Serum thyroglobulin and recurrent thyroid cancer. Lancet. 1977;1(8017):881–2. doi: 10.1016/s0140-6736(77)91202-8. [DOI] [PubMed] [Google Scholar]
- 10.Ruter A, Smeds S, Lennquist S. Value of serum thyroglobulin measurement in patients operating on for well differentiated thyroid carcinoma. Eur J Surg. 1998;164(9):665–71. doi: 10.1080/110241598750005543. [DOI] [PubMed] [Google Scholar]
- 11.Kashat L, Orlov S, Orlov D, Assi J, Salari F, Walfish PG. Serial post-surgical stimulated and unstimulated highly sensitive thyroglobulin measurements in low- and intermediate-risk papillary thyroid carcinoma patients not receiving radioactive iodine. Endocrine. 2016;54(2):460–6. doi: 10.1007/s12020-016-0989-3. [DOI] [PubMed] [Google Scholar]
- 12.Prior-Sanchez I, Barrera Martin A, Moreno Ortega E, Vallejo Casas JA, Galvez Moreno MA. Is a second recombinant human thyrotropin stimulation test useful? The value of postsurgical undetectable stimulated thyroglobulin level at the time of remnant ablation on clinical outcome. Clin Endocrinol (Oxf) 2017;86(1):97–107. doi: 10.1111/cen.13140. [DOI] [PubMed] [Google Scholar]
- 13.Janovsky CC, Maciel RM, Camacho CP, Padovani RP, Nakabashi CC, Yang JH, et al. A Prospective Study Showing an Excellent Response of Patients with Low-Risk Differentiated Thyroid Cancer Who Did Not Undergo Radioiodine Remnant Ablation after Total Thyroidectomy. European thyroid journal. 2016;5(1):44–9. doi: 10.1159/000442048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HS, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Risk Factors for Re-recurrence After First Reoperative Surgery for Locoregional Recurrent/Persistent Papillary Thyroid Carcinoma. World journal of surgery. 2015;39(8):1943–50. doi: 10.1007/s00268-015-3052-2. [DOI] [PubMed] [Google Scholar]
- 15.Yim JH, Kim WB, Kim EY, Kim WG, Kim TY, Ryu JS, et al. The outcomes of first reoperation for locoregionally recurrent/persistent papillary thyroid carcinoma in patients who initially underwent total thyroidectomy and remnant ablation. J Clin Endocrinol Metab. 2011;96(7):2049–56. doi: 10.1210/jc.2010-2298. [DOI] [PubMed] [Google Scholar]
- 16.Rosario PW, Mourao GF, Calsolari MR. Can the follow-up of patients with papillary thyroid carcinoma of low and intermediate risk and excellent response to initial therapy be simplified using second-generation thyroglobulin assays? Clinical endocrinology. 2016;85(4):596–601. doi: 10.1111/cen.13053. [DOI] [PubMed] [Google Scholar]
- 17.Choudhary C, Wartofsky L, Tefera E, Burman KD. Evaluation of Thyroid Bed Nodules on Ultrasonography after Total Thyroidectomy: Risk for Loco-Regional Recurrence of Thyroid Cancer. European thyroid journal. 2015;4(2):106–14. doi: 10.1159/000431317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Bergren R, Schneider D, Chen H, Sippel RS. Thyroglobulin antibody resolution after total thyroidectomy for cancer. J Surg Res. 2015;198(2):366–70. doi: 10.1016/j.jss.2015.03.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh CJ, Wang PW. Sequential changes of serum antithyroglobulin antibody levels are a good predictor of disease activity in thyroglobulin-negative patients with papillary thyroid carcinoma. Thyroid. 2014;24(3):488–93. doi: 10.1089/thy.2012.0611. [DOI] [PubMed] [Google Scholar]
- 20.Miah CF, Zaman JA, Simon M, Davidov T, Trooskin SZ. The utility of lymph node mapping sonogram and thyroglobulin surveillance in post thyroidectomy papillary thyroid cancer patients. Surgery. 2014;156(6):1491–6. doi: 10.1016/j.surg.2014.08.054. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 21.Oltmann SC, Schneider DF, Chen H, Sippel RS. All thyroid ultrasound evaluations are not equal: sonographers specialized in thyroid cancer correctly label clinical N0 disease in well differentiated thyroid cancer. Annals of surgical oncology. 2015;22(2):422–8. doi: 10.1245/s10434-014-4089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poehls JL, Chen H, Sippel RS. Preoperative ultrasonography findings predict the need for repeated surgery in papillary thyroid cancer. Endocr Pract. 2012;18(3):403–9. doi: 10.4158/EP11221.OR. [DOI] [PubMed] [Google Scholar]
- 23.Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph node ratio predicts recurrence in papillary thyroid cancer. The oncologist. 2013;18(2):157–62. doi: 10.1634/theoncologist.2012-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson TJ, Thomas S, Dinan MA, Roman S, Sosa JA, Hyslop T. How Many Lymph Nodes Are Enough? Assessing the Adequacy of Lymph Node Yield for Papillary Thyroid Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(28):3434–9. doi: 10.1200/JCO.2016.67.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onkendi EO, McKenzie TJ, Richards ML, Farley DR, Thompson GB, Kasperbauer JL, et al. Reoperative experience with papillary thyroid cancer. World J Surg. 2014;38(3):645–52. doi: 10.1007/s00268-013-2379-9. [DOI] [PubMed] [Google Scholar]