Abstract
Objective
The frequency of mind-wandering decreases as a function of age in healthy individuals. One possible explanation is that mind-wandering is a resource-dependent process, and cognitive resources decline with age. The present study provides the first investigation of mind-wandering in the earliest stages of Alzheimer’s disease (AD) to further examine the resource model and discontinuities between healthy aging and AD.
Methods
Three large cohorts completed the Sustained Attention to Response Task (SART): a healthy middle-aged group (mean age = 61.79 ± 5.84 years; N = 270), a healthy older adult group (mean age = 76.58 ± 5.27 years; N = 282), and a group with early stage AD (mean age = 76.08 ± 7.17; N = 77), comparable in age to the second group.
Results
Self-reports of mind-wandering during the SART decreased as a function of age, and there was a further decrease in the AD group. All three groups produced faster responses on trials before No-Go errors, suggesting mind-wandering occurred in all cohorts. After No-Go errors, healthy older adults slowed disproportionately compared to middle-aged adults. This was not evident in AD individuals who showed post-error slowing comparable to that in the middle-aged group.
Conclusions
The decreased self-reported mind-wandering in older adults and the further decline in AD are consistent with the cognitive resource account of mind-wandering. Behavioral indices suggest that AD is on a continuum with healthy aging, with the exception of post-error slowing which may suggest performance monitoring deficits in early AD individuals (e.g., lack of error awareness).
Keywords: mind-wandering, SART, aging, attention, Alzheimer’s disease
Mind-wandering (MW) refers to the phenomenon of attention shifting away from mental contents related to the task at hand to unrelated thoughts and feelings (Christoff, Irving, Fox, Spreng, & Andrews-Hanna, 2016; Smallwood & Schooler, 2006; 2015), and is considered to be a specific mental state within a spectrum of spontaneous thought phenomena (Christoff et al., 2016). There is evidence that people spend a considerable amount of time mind-wandering in their everyday lives and therefore it appears to be a prominent feature of human phenomenology (Giambra, 1989; Killingsworth & Gilbert, 2010; Kane et al., 2007; McVay, Kane, & Kwapil, 2009; Singer & McCraven, 1961; Stawarczyk, Majerus, Maj, Van der Linden, & D’Argembeau, 2011).
Methods developed to tap MW often include some form of thought sampling (Smallwood & Schooler, 2006). For example, researchers often use an experience sampling approach which probes individuals intermittently during their everyday activities to determine if the participant is thinking about his or her current task or not (see e.g., Kane et al., 2007; Killingsworth & Gilbert, 2010). In laboratory studies, thought sampling reflects participants responding to a number of probe questions that usually appear randomly or quasi-randomly throughout an experimental task (Smallwood & Schooler, 2006, 2015). These questions prompt individuals to report their mental contents at the time of the probe. In addition to random sampling, studies sometimes utilize reports of “self-caught mind-wandering” (Smallwood & Schooler, 2006). In this case, participants have to press a key during task performance whenever they detect that their attention has shifted away from the primary task (e.g., Cunningham, Scerbo, & Freeman, 2000; Jackson & Balota, 2012; Jackson, Weinstein, & Balota, 2013; Sayette, Reichle, & Schooler, 2009). For example, this might occur when an individual is reading and realizes that he/she has been thinking of something other than the material. This method, however, is unable to detect MW that happens in the absence of meta-awareness (i.e., when participants do not notice that their mind is wandering), even though such meta-awareness is not required for MW to occur (Schooler, Reichle, & Halpern, 2004; Schooler et al., 2011; Smallwood, McSpadden, & Schooler, 2007, 2008; Smallwood & Schooler, 2006).
In addition to subjective reports of MW, a common task used to indirectly measure MW is the Sustained Attention to Response Task (SART, e.g., Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Smallwood et al., 2004; Smallwood, Beach, Schooler, & Handy, 2008; Stawarczyk, Majerus, Maj, et al., 2011) in which participants have to respond to frequent non-targets (Go trials), but have to withhold their response to infrequent targets (or No-Go trials; Robertson, Manly, Andrade, Baddeley, & Yiend, 1997; Smallwood et al., 2004). In addition to self-report data from thought probes embedded within the task, the SART also provides reaction-time (RT) based indices of MW. Two typical RT-based measures of MW in the SART are pre-error speeding and post-error slowing. Pre-error speeding refers to the observation that response latencies on Go trials preceding a No-Go error (i.e., responding to a No-Go target) are generally shorter than on trials preceding correct No-Go trials, presumably reflecting “mindless” rhythmical responding (Smallwood & Schooler, 2006). Post-error slowing refers to the relative slowing of responses following an incorrect No-Go trial compared to responses following a correct No-Go trial. This presumably reflects task re-engagement following the detection of an error caused by a MW episode or attentional lapse (Cheyne, Carriere, Solman, & Smilek, 2011; Cheyne, Solman, Carriere, & Smilek, 2009; Jackson & Balota, 2012; McVay, Meier, Touron, & Kane, 2013). A further behavioral indicator is RT variability as indexed by the coefficient of variation (CoV = SD of Go RTs/Mean Go RTs) (Cheyne et al., 2009). Greater cross-trial intraindividual variability presumably reflects more frequent episodes of transient (partial or complete) disengagement from the task. Importantly, however, RT-based measures may have some limitations. For instance, pre-error speeding could be produced by multiple factors in addition to – or instead of – MW, such as a build-up of a motor habit or a speed-accuracy trade-off (McVay & Kane, 2012a). Nevertheless, we still consider behavioral indices important as they are not limited to the time points directly preceding randomly distributed probes and they do not rely on participants’ metacognitive abilities (i.e., participants might misclassify their mental contents).
Mind-wandering and aging
The relationship between healthy aging and MW has been the focus of considerable work utilizing the methods outlined above (for a recent review see Maillet & Schacter, 2016a). Irrespective of the method used, a relatively consistent, and somewhat surprising, pattern emerges in the literature: older adults tend to report less MW than younger adults (Giambra, 1989; 2000; Jackson & Balota, 2012; Jackson et al., 2013; Krawietz, Tamplin, & Radvansky, 2012; Staub, Doignon-Camus, Bacon, & Bonnefond, 2014; Shake, Shulley, & Soto-Freita, 2015; Zavagnin, Borella, & De Beni, 2014). In addition, older adults do not seem to show differential pre-error speeding, but there is an age-related increase in post-error slowing, which may suggest that task re-engagement following periods of MW require greater effort from older adults than from younger adults, or that older adults devote more attention to evaluating their performance on the task (see for example, Jackson & Balota, 2012; McVay et al., 2013 for discussions).
There are a number of factors that can account for the decrease in MW with age. First, according to Smallwood and Schooler (2006), MW requires cognitive resources because it involves executive control shifting from task performance to personal goals. Because cognitive resources decline with age (Craik, 1986; Salthouse, 2009), older adults engage in MW less than younger adults. However, it should be noted that in the study by Shake et al. (2015) cognitive abilities (e.g., working memory capacity) were not associated with MW after controlling for age and interest. This lack of a relationship is somewhat difficult to reconcile with the cognitive resource account of MW. A similar account suggests that older adults may simply find the tasks more engaging and interesting, and the reduction in MW is merely a product of this absorption. Jackson and Balota (2012), Krawietz et al. (2012), and Shake et al. (2015) all reported that older adults found the tasks used in these studies (i.e., the SART and reading tasks) more interesting, and that interest was a predictor of MW. Jackson and Balota (2012) also found that older adults were more conscientious than younger adults, and thus less frequent MW in the former group may have been the result of a more disciplined attitude towards task completion. A third account of the age-related decrease in self-reported MW posits that older adults have fewer environmentally-triggered concerns than younger adults, and consequently may have fewer competing thoughts that could draw their attention away from task performance (Giambra, 1993; McVay & Kane, 2010; Parks, Klinger, & Perlmutter, 1988). Finally, it is also possible that older adults are simply reluctant - or unable - to accurately report their experiences when prompted by the probes (e.g., they misclassify off-task thought as on-task). To investigate this latter account, Frank, Nara, Zavagnin, Touron, and Kane (2015) recently tested the validity of older adult thought reports using eye-tracking during a reading comprehension task. Results showed that eye movements preceding probes differentiated between on-task and off-task thought reports (MW) in both older and younger adults. This pattern was interpreted as indicating that older adults are indeed just as good as younger adults in reporting MW.
Present research
Although there has been considerable work investigating the intriguing finding that older adults consistently report less MW than younger adults, to our knowledge, there has yet to be any study of older adults who are transitioning to the earliest stages of Alzheimer’s disease (AD). This is the focus of the present study. AD pathology involves breakdowns in a number of cognitive processes, most notably episodic memory (Albert, Moss, Blacker, Tanzi, & McArdle, 2007; Storandt, Grant, Miller, & Morris, 2006) and executive/attentional processes (Balota & Faust, 2001; Balota et al., 2010; Faust & Balota, 2007). Consequently, the cognitive resource account of MW would suggest that older individuals with very mild dementia might report even less MW than cognitively healthy older adults because they have fewer resources left over for the executive control of MW. It is worth noting, however, that Duchek, Balota, Storandt, and Larsen (2007) found that very mildly demented individuals are also less conscientious than cognitively healthy controls, and so one might expect that individuals in the earliest stages of AD might show more MW than more conscientious healthy controls, if indeed conscientiousness is related to MW reports.
Furthermore, individuals with early stage AD are a particularly intriguing group to study because of the accumulating evidence indicating that there is a break-down in the “default mode network” in early stage AD (Greicius, Srivastava, Reiss, & Menon, 2004; Lee et al., 2016; Lustig et al., 2003), which may be related to MW reports. The default mode network (DMN) is a set of brain regions that is active during periods of passive rest and becomes deactivated during task performance (Buckner, 2012; Raichle et al., 2001; Shulman et al., 1997). Importantly, DMN activity is also thought be associated with spontaneous cognition, such as in MW (Andrews-Hanna, Reidler, Huang, & Buckner, 2010; Christoff et al., 2016; Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015; Mason et al., 2007; for studies specifically focusing on MW during SART see Christoff et al., 2009; Durantin, Dehais, & Delorme, 2015; Kirschner, Kam, Handy, & Ward, 2012; Stawarcyzk, Majerus, Maquet, & D’Argembeau, 2011). Because AD pathology disrupts the functioning of a network implied in MW, individuals showing early signs of AD may produce less MW than healthy controls. On the other hand, because there is evidence suggesting that the DMN is less deactivated during task performance in AD individuals than in healthy control individuals (Lustig et al., 2003), one may actually find more mind-wandering during task performance in the former group. The present study will provide evidence regarding these contrasting predictions of MW in healthy and abnormal aging at the behavioral level.
The present study included three large groups of well-characterized older adults (N = 629) who actively participate in longitudinal studies: two groups of cognitively healthy older adults (a younger group of subjects, mean age = 61.79, and an older group of participants, mean age = 76.58), and a group of older adults at the earliest detectable stage of AD (comparable in age to the older healthy control group, mean age = 76.08). Participants completed the SART with random thought probes. In addition to the thought probes, we examined the behavioral indices of MW (pre-error speeding, post-error slowing, and the coefficient of variation). Based on Jackson and Balota’s (2012) results, we expected to find larger post-error slowing as a function of age. This would be an important extension of earlier results because the vast majority of the aging studies of mind wandering have compared young college students to healthy older adults. In the present study, we compared a young-old group with an older-old group to examine if one can detect differences in post-error slowing between the two healthy community dwelling older groups that do not have extreme age differences. If we replicate the previous studies in this older sample, we also expected to find fewer MW reports in the old-old participants than the young-old participants.
Importantly, the present study also examined individuals with early stage AD. If AD is on a continuum with healthy aging, then one might expect a further increase in post-error slowing in the AD group as task re-engagement might prove most difficult for participants with attentional control deficits beyond age-related decline. Predictions for pre-error speeding are less clear as age did not disproportionately affect this index in Jackson and Balota’s (2012) original study. In addition, one may expect fewer subjective reports of MW in the early stage AD individuals in accordance with the predictions of the cognitive resource account. However, one could also expect an increased reporting of MW in the early stage AD individuals, compared to healthy older adults, if conscientiousness underlies the decreased reporting of MW in older adults compared to younger adults. This prediction is based on the decrease in conscientiousness in the early stages of AD compared to healthy older adults (e.g., Duchek et al., 2007),
Method
Participants
A sample of 629 individuals participated in this study: 552 cognitively healthy adults (Clinical Dementia Rating; CDR = 0, no dementia) and 77 older adults in the earliest stages of AD (CDR higher than 0). Out of these individuals, 49 had a CDR of .5 (very mild dementia) and 28 had a CDR of 1 (mild dementia, see Morris, 1993). Healthy participants were further divided into middle-aged adults (age ≤ 69; Mage = 61.79, SD = 5.84; range = 43 – 69; n = 270; 63.7% female) and older adults (age > 69; Mage = 76.58, SD = 5.27; range = 70 – 94; n = 282; 59.6% female) to examine the effects of healthy aging. The individuals in the early stages of AD were grouped together regardless of age (Mage = 76.08, SD = 7.17; range = 63 – 95; n = 77; 44.2% female), but their mean age was not significantly different from that of the older adult group (Welch test, F < .05; p = .569).
All participants were recruited from the Charles and Joanne Knight Alzheimer’s Disease Research Center (Knight ADRC) as part of either the Healthy Aging & Senile Dementia (HASD) project or the Adult Children Study (ACS). Both projects are longitudinal in design and follow individuals who are cognitively healthy upon entering the study. Participants are first screened for other forms of cognitive impairment (e.g., depression, hypertension, etc.) to be consistent with the criteria for “probable AD” as determined by the National Institute of Neurological and Communication Disorders and Stroke—Alzheimer’s Disease and Related Disorders Association (McKhann et al., 1984). Dementia ratings are then assessed using the Washington University CDR scale (Morris, 1993), which assesses dementia severity with CDR 0, 0.5, 1, 2, and 3, corresponding to no dementia, very mild, mild, moderate, and severe dementia, respectively. The CDR assessment encompasses a 90-minute clinical interview that assesses changes in cognition and participant functioning in such domains as memory, orientation, problem solving, community involvement, and personal care; and gathers information from a close collateral source (e.g., family member) too. The reliability and validity of the diagnosis at autopsy have been quite high (93% accuracy), even for those with very mild AD (Storandt et al., 2006). Importantly, only individuals diagnosed with symptomatic AD were included in this study, and so we excluded all individuals with non-AD, uncertain or mixed etiologies, such as frontotemporal contributions to dementia. If a subject completed the SART more than once, only their baseline SART performance was used in the present analyses. Information about CDR status at the time of the SART was also obtained for all participants. Where available, personality data assessed by the NEO-FFI (Costa & McCrae, 1992) was also included.
Table 1 provides demographic data as a function of group. Participants also completed a battery of psychometric tests as part of the project they were enrolled in. These tests were administered during a separate session. The table includes results for the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), the Selective Reminding Test (SRT; Buschke, 1973) and the reading span task (Daneman & Carpenter, 1980). Psychometric data for a given participant was only included if it was collected no later than +/− 1 year from the date of SART completion. As shown in Table 1, performance significantly declined across groups from younger adults to older adults, and from older adults to early AD (ps < .05 for all group wise comparisons).
Table 1.
Demographic and psychometric data – means (SDs) - as a function of group.
| Healthy middle-aged | Healthy older adults | AD individuals | ||||
|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Age (years) | 270 | 61.79 (5.84) | 282 | 76.58 (5.27) | 77 | 76.08 (7.17) |
| Education (years) | 261 | 16.13 (2.52) | 269 | 15.40 (2.68) | 71 | 15.55 (2.88) |
| MMSE | 236 | 29.32 (.92) | 267 | 28.86 (1.45) | 71 | 24.92 (3.53) |
| SRT - Free Recall | 229 | 32.89 (5.19) | 259 | 31.42 (6.16) | 52 | 16.17 (9.44) |
| SRT - Total Recall | 229 | 47.90 (.32) | 259 | 47.76 (.64) | 52 | 40.58 (9.17) |
| Reading Span | 179 | 8.04 (2.76) | 178 | 6.35 (2.26) | 50 | 4.52 (1.98) |
Note: MMSE = Mini-Mental State Examination; SRT = Selective Reminding Test. Higher scores indicate better performance.
Materials
In the SART, participants were presented with single numbers 1–9 with the number 3 identified as a target (No-Go) stimulus to which they should withhold their response. If any other digit (non-target, or Go stimulus) appeared, participants were required to press the SPACE bar. The task consisted of 125 trials, and lasted approximately 5 minutes. Each digit was presented for 1250 ms followed by a blank screen of the same duration. Targets were presented on 11.1% of the trials. Throughout the task, 5 thought probes appeared in a random fashion.
Procedure
SART sessions were preceded by 3 blocks of practice. In the first practice block, 9 trials (1 target) were presented with feedback, without any random thought probes. The second practice block was identical, with the exception that a single thought probe was also presented. In the third practice block, participants no longer received feedback on their performance. With this exception, it was identical to the preceding blocks. This was followed by a test block of 125 trials.
Stimuli were presented centrally, in white against a black background in 44 pt Courier New font. On probe trials, subjects saw the following instruction: “Please choose the one option below which best describes your experience with the task just now”. They then indicated their response by pressing a number key corresponding to one of the following categories: 1) I was thinking about the task; 2) My mind was blank; 3) My mind drifted to things other than the task, but I wasn’t aware of it until you asked me; 4) While doing the task I was aware that thoughts about other things popped into my head. These reflect on-task thought, space outs, zone outs, and tune outs, respectively (Jackson & Balota, 2012; Smallwood, McSpadden, & Schooler, 2007). Upon completion of the task, participants also indicated how difficult and how interesting they found the task on a 5-point Likert scale. The experiment was run using E-Prime 1.2 Software (Psychology Software Tools, Pittsburgh, PA).
Results
An alpha of .05 was set to indicate significance in all analyses. Mean response latencies and accuracies as a function of group (middle-aged, healthy older adult, AD) are presented in Table 2. Trial level RTs below 150 ms and above 2000 ms were removed prior to analyses (.04% of trials were removed due to this screening). Following this, RTs beyond three standard deviations of a participant’s mean were also removed. This resulted in the removal of 1.75% of trials in the middle-aged group, 1.87% in the healthy older adult group, and 1.72% in the AD group1. In addition, analyses are also reported on standardized RTs, based on the subject’s mean and standard deviation in order to control for general slowing across groups (see Faust, Balota, Spieler, & Ferraro, 1999). Corresponding z-scores are also presented in Table 2.
Table 2.
Descriptive data – means (SDs) – for middle-aged adults, healthy older adults, and early stage AD individuals for the SART.
|
Healthy middle- aged |
Healthy older adults |
AD individuals | |
|---|---|---|---|
| Accuracy | |||
| N | 270 | 282 | 77 |
| Go accuracy | .99 (.01) | .99 (.02) | .95 (.06) |
| No-Go accuracy | .95 (.07) | .94 (.08) | .90 (.12) |
| Go Reaction Time | |||
| N | 270 | 282 | 77 |
| Go RT | 499.80 (75.19) | 532.87 (74.06) | 563.16 (95.61) |
| Go Zrt | .01 (.01) | .01 (.01) | .01 (.01) |
| No-Go Reaction Time | |||
| N | 127 | 132 | 45 |
| No-Go RT | 403.15 (110.64) | 437.87 (120.56) | 433.49 (116.86) |
| No-Go zRT | −.85 (.97) | −.82 (.94) | −.93 (.64) |
| Performance indices | |||
| N | 270 | 282 | 77 |
| Go RT CV | .19 (.04) | .19 (.04) | .23 (.06) |
| Skill index | 1.93 (.30) | 1.80 (.27) | 1.64 (.35) |
| Pre-error speeding | |||
| N | 82 | 88 | 28 |
| N-4 No-Go Error RT | 432.70 (80.74) | 479.53 (84.55) | 489.55 (107.64) |
| N-4 No-Go Error | |||
| zRT | −.47 (.55) | −.37 (.52) | −.47 (.51) |
| N-4 No-Go Correct | |||
| RT | 480.57 (70.85) | 516.06 (76.32) | 548.09 (86.90) |
| N-4 No-Go Correct | |||
| zRT | .02 (.21) | −.04 (.20) | −.03 (.16) |
| Post-error slowing | |||
| N | 109 | 110 | 41 |
| N+1 No-Go Error RT | 532.98 (144.53) | 597.64 (146.29) | 623.01 (131.92) |
| N+1 No-Go Error | |||
| zRT | .46 (1.11) | .84 (1.18) | .48 (.74) |
| N+1 No-Go Correct | |||
| RT | 457.95 (84.71) | 481.49 (90.13) | 522.42 (105.15) |
| N+1 No-Go Correct | |||
| zRT | −.29 (.43) | −.31 (.42) | −.28 (.50) |
Note: Because not all participants committed No-Go errors, reaction times for pre- and post-correct RTs are reported from the subsample of those participants who had pre- or post-error RT data.
Following Jackson and Balota (2012), thought probes that occurred immediately following a No-Go target due to the randomization were removed from later analyses, since rare No-Go trials may engage task attention. Approximately 11.77% of probes were lost due to this screening. In addition, data from 7 participants (N = 7) who only had 1 or 2 probe responses due to this randomization were not analyzed.
Accuracy
In order to investigate the effects of Age and CDR status on accuracy, a 2 (Go trial accuracy vs. No-Go trial accuracy) × 3 (healthy middle-aged, healthy old, AD) mixed model ANOVA was conducted. Results indicated that subjects were significantly more accurate on Go trials than on No-Go trials (F(1,626) = 140.64, p < .001). A main effect of group was also found (F(2,626) = 35.92, p < .001) indicating that there was no difference in accuracy between the two healthy groups, but AD individuals were significantly less accurate than both other groups (both ps < .001). There was no interaction between group and trial type (F < 1).
Response latencies
A 2 (No-Go response latency vs. Go response latency) × 3 (healthy middle-aged, healthy old, or AD) mixed model ANOVA was conducted to examine the effects of CDR status on reaction times. Subjects were faster on No-Go error trials than on correct Go trials (F(1,301) = 238.00, p < .001). Response latencies varied as a function of group (F(2,301) = 8.10, p < .001): middle-aged adults were significantly faster than both older groups (ps < .01), however, there was no significant difference between healthy and AD older adults.2 There was a significant group by trial type interaction (F(2,301) = 3.70, p < .05). In follow-up analyses, separate Group × Trial Type ANOVAs indicated that there was a significantly greater difference between No-Go and Go RTs in AD participants, compared to middle-aged adults (F(1,170) = 6.31, p < .05), and in AD participants compared to the age-matched older adults (F(1,175) = 6.53, p < .05). When z-scores were analyzed instead of raw reaction times, No-Go error z-RTs were still significantly faster than Go z-RTs (F(1,301) = 217.69, p < .001), but neither the main effect of group nor the group by trial type interaction was reliable (Fs < 1).
Additional measures from the SART
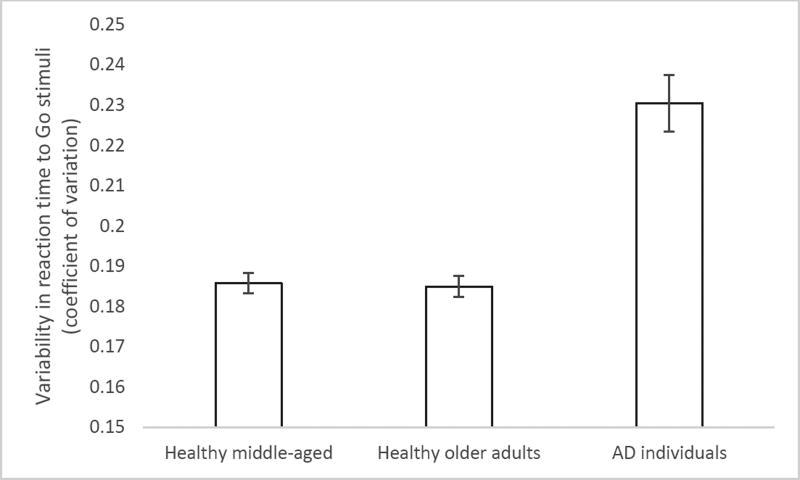
In order to investigate intra-individual variability across groups, the coefficient of variation (CoV) was calculated for each participant by dividing the standard deviation of their Go RTs by their mean Go RT. Since the assumption of homogeneity of variances was not met, a Welch test was run to examine group differences in CoV. CoV was reliably different across groups (F(2,195.74) = 19.38, p < .001). According to post-hoc analysis, participants in the early stages of AD showed significantly more variable performance (higher CoV) than did those in other two groups (p < .001). The healthy groups did not differ from each other. This result is illustrated in Figure 1.
Figure 1.
Reaction time variability (coefficient of variation) as a function of group. Error bars represent +/− 1 SE.
A skill index (Seli, 2016, see also Seli, Jonker, Solman, Cheyne, & Smilek, 2013) was also calculated. This index controls for individual differences in speed-accuracy trade-offs, and is calculated by dividing each subject’s mean No-Go accuracy by their mean Go RT. The result is then multiplied by 1000 for interpretive convenience (Seli, 2016). Higher skill index indicates better performance on the SART. Skill index systematically decreased as a function of both healthy aging and early stage AD (Welch test, F(2,200.83) = 27.93, p < .001), with each group being reliably different from each other (all ps < .01).
Pre-error speeding
In the following analyses the mean response latency of four Go trials preceding a No-Go error (i.e., when a response was made to a target) is contrasted against the mean response latency of four Go trials preceding a correct No-Go trial (i.e., where no response was made). If MW is occurring, one might expect trials before an error to be relatively fast because participants are not engaged in fully processing these items, and in some sense are mindlessly pressing the button. Indeed, a 2 (Pre-error RT vs. Pre-correct RT) × 3 (healthy middle-aged, healthy old, or AD) mixed-model ANOVA indicated that trials preceding No-Go errors were faster than trials preceding correctly withheld No-Go responses (F(1,195) = 75.47, p < .001). The main effect of group was also significant (F(2,195) = 10.24, p < .001) as middle-aged adults were faster than both healthy older adults and AD individuals. Results showed no group by trial type interaction (F < 1.5). Turning to the z-transformed RTs, there was a reliable main effect of trial type (F(1,195) = 70.37, p < .001), but neither the main effect of group nor the group by trial type interaction approached significance (Fs < 1.5) in this pre-error speeding analysis.
Post-error slowing
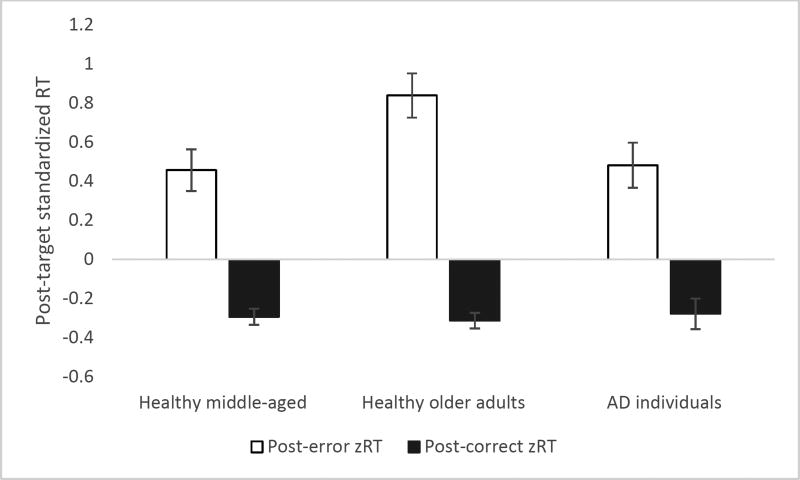
Post-error reaction time refers to the response latency on the Go trial immediately following a No-Go error, whereas post-correct reaction time refers to the response latency on the trial following a correctly withheld No-Go response. It is hypothesized that post-error slowing reflects re-engaging the appropriate task set after an error is made. Reaction time patterns were analyzed in a 2 (Post-error vs. Post-correct) × 3 (healthy middle-aged, healthy old, AD) mixed-model ANOVA. Post-error response latencies were longer than post-correct response latencies (F(1,257) = 138.87, p < .001). Group also produced a significant main effect (F(2,257) = 9.80, p < .001): healthy middle-aged adults were faster than the other two groups. A significant group by trial type interaction was also found (F(2,257) = 3.25, p < .05). To fully explore the nature of this interaction, three separate 2 × 2 mixed-model ANOVAs were conducted. In the first analysis, only the two healthy groups were contrasted. This revealed significant main effects of trial type (F(1,217) = 135.54, p < .001) and age group (F(1,217) = 9.94, p < .01), and, most importantly, a significant group by trial type interaction (F(1,217) = 6.27, p < .05). This indicates that the difference between post-error and post-correct RTs was greater in the older group than in the younger, replicating and extending the pattern observed by Jackson and Balota (2012) when comparing young college students to older adults. In the second ANOVA, only the healthy older adults and the AD individuals were contrasted. A significant main effect of trial type (F(1,149) = 101.03, p < .001) and a marginal main effect of group (F(1,149) = 2.95, p = .088) were found, however, the group by trial type interaction was not reliable (F < 1). In the final ANOVA, the healthy middle-aged group was contrasted with the AD group. This indicated significant main effects of trial type (F(1,148) = 64.34, p < .001) and group (F(1,148) = 16.91, p < .001), but the group by trial type interaction was not reliable in this analysis either (F < 1.5). In sum, the results of the follow-up analyses suggest that healthy older adults were slowing disproportionately compared to younger healthy adults (i.e., the difference between post-error and post-correct RTs was bigger), but this tendency was not evident in AD individuals as there was no reliable difference between middle-aged and AD - or older and AD - adults.
Turning to the z-score analyses, there was a reliable main effect of trial type (F(1,257) = 125.83, p < .001) indicating slowing after errors and a marginal main effect of group (F(2,257) = 2.69, p = .070). Importantly, the group by trial type interaction was significant (F(2,257) = 3.83, p < .05). We conducted follow-up analyses on z-scores in a fashion similar to the analyses for the raw RTs. In the first ANOVA contrasting the healthy groups (middle-aged and older), a significant main effect of trial type (F(1,217) = 136.60, p < .001) and group (F(1,217) = 4.64, p < .05) were found. The group by trial type interaction was also reliable (F(1,217) = 6.11, p < .05). The second ANOVA contrasting the AD participants and the age-matched controls revealed a significant main effect of trial type (F(1,149) = 87.51, p < .001), but the main effect of group did not reach significance (F < 2.5). The group by trial type interaction was marginally significant (F(1,149) = 3.68, p = .057) suggesting that post-error slowing is slightly smaller in the AD individuals compared to their age-matched controls, when controlling for overall processing speed. In the final ANOVA contrasting healthy middle-aged adults and CDR .5 and 1 individuals, only a main effect of trial type was found (F(1,148) = 56.45, p < .001), with neither the main effect of group nor the interaction between group and condition approaching significance (both Fs < .5). These results corroborate the results of the analyses on raw RTs.
Self-reported mind-wandering
Mean proportions of different thought probe responses (on-task thought, space outs, zone outs, and tune outs) in each group are presented in Table 3.
Table 3.
Mean proportions (SDs) of different thought reports across groups.
| Healthy middle-aged | Healthy older adults | AD individuals | |
|---|---|---|---|
| N | 270 | 282 | 77 |
| On-task | .63 (.33) | .72 (.32) | .88 (.24) |
| Space outs | .02 (.09) | .03 (.12) | .03 (.10) |
| Zone outs | .08 (.15) | .07 (.15) | .04 (.13) |
| Tune outs | .26 (.30) | .18 (.27) | .05 (.13) |
Because the distributions of thought report frequencies were skewed, Kruskal-Wallis H tests were used to examine the effect of age/CDR status on mind-wandering. Frequency of on-task thought reports was reliably different across groups (χ2 (2) = 43.48, p < .001). Pairwise comparisons revealed that each group was significantly different from every other group (ps < .01). In other words, middle-aged adults reported less on-task thoughts than older adults who, in turn, reported less than AD individuals. Frequency of tune outs (mind-wandering with awareness) was also different across groups (χ2 (2) = 43.94, p < .001). All three pairwise comparisons yielded significant results (ps < .01), thus tune outs decreased in frequency with age, and showed further decrease in the AD group. No compelling significant differences were found in the remaining two thought report categories (space outs and zone outs).3
Personality, interest, difficulty, and working memory capacity
When the five factors of personality were examined, ANOVAs revealed significant differences between groups in Neuroticism (F(2,559) = 9.48, p < .001), Openness (F(2,559) = 18.31, p < .001), and Conscientiousness (F(2,559) = 8.17, p < .001). See Table 4 for descriptive data.
Table 4.
Descriptive data – means (SDs) – for middle-aged adults, healthy older adults, and early stage AD individuals for personality dimensions, and interest and difficulty ratings.
| Healthy middle-aged | Healthy older adults | AD individuals | ||
|---|---|---|---|---|
| Personality | ||||
| N | 245 | 248 | 69 | |
| Neuroticism | 15.51 (7.84) | 14.15 (7.00) | 18.42 (6.39) | |
| Extraversion | 29.98 (6.79) | 29.60 (6.24) | 28.58 (5.18) | |
| Openness | 30.09 (5.99) | 27.59 (5.91) | 25.99 (5.18) | |
| Agreeableness | 34.71 (5.76) | 35.20 (5.02) | 34.32 (4.96) | |
| Conscientiousness | 35.23 (6.57) | 34.53 (6.32) | 31.64 (7.11) | |
| Task ratings | ||||
| N | 270 | 282 | 77 | |
| Interest | 2.21 (1.10) | 2.50 (1.10) | 2.94 (1.19) | |
| Difficulty | 1.53 (.78) | 1.63 (.75) | 2.03 (1.04) |
The significant difference in Neuroticism scores was driven by significant differences between the AD group and the two cognitively healthy groups (ps ≤ .010) with no reliable difference between the latter two groups (p = .095). In the case of Openness, healthy middle-aged adults scored significantly higher than both older groups (ps < .001); there was no significant difference between cognitively healthy older adults and the AD group (p = .111). AD individuals were significantly less conscientious than healthy individuals regardless of age (ps < .01), and there was no reliable difference between the two healthy groups. These patterns replicate Duchek et al. (2007).
Investigating the relationship between Conscientiousness and SART performance controlling for CDR status, we found that Conscientiousness was negatively correlated with CoV (β = − .16, p < .001), and mean Go RT (β = − .09, p < .05), and positively with skill (β = .11, p < .01). However, conscientiousness was unrelated to self-reported mind-wandering (rho = .07 for on-task thought, and rho = −.06 for tune outs) using Spearman’s rho which does not control for covariates.
Interest ratings differed significantly across groups (χ2 (2) = 25.21, p < .001). Each group differed significantly from every other group (ps < .05). Middle-aged adults found the task the least interesting, while the AD group found the SART significantly more interesting than the two healthy older adult groups. Difficulty ratings were also significantly different across groups (χ2 (2) = 18.80, p < .001). The AD group rated the task more difficult than the two healthy groups (ps < .01). Interest and difficulty ratings were positively correlated in the sample (rho = .27, p < .001). Interest was weakly positively correlated with mean Go RT (rho = .09, p < .01), and on-task thought reports (rho = .19, p < .001). It was negatively correlated with tune outs (rho = − .16, p < .001). Perceived difficulty was positively correlated with CoV (rho = .17, p < .001). It was negatively correlated with No-Go accuracy (rho = − .30, p < .001), skill (rho = − .22, p < .001), and tune out frequency (rho = − .09, p < .05).
We also examined the relationship between MW reports and working memory. Across all participants and groups, on-task thought frequency was negatively correlated with reading span (rho = − . 18, p < .001), while tune out frequency was positively correlated with reading span (rho = .21, p < .001). However, neither of the correlations remained significant within groups. When group differences in MW were analyzed with reading span entered as a covariate, significant differences were still found both for on-task thoughts (F(2,403) = 5.45, p < .01) and for tune outs (F(2,403) = 6.50, p < .01).
Discussion
The goal of the present study was to investigate mind-wandering (MW), as reflected by SART performance and self-reports, as a function of healthy aging and in early stage AD. Self-reports of mind-wandering decreased across groups with the youngest healthy group reporting the most off-task thought, older healthy adults reporting significantly less (replicating previous findings, see Maillet & Schacter, 2016a), and the AD group reporting the least. With respect to behavioral indices of MW in the SART, no group differences were observed in pre-error speeding after adjusting for processing speed, but there were increases in the older adults compared to younger adults in the coefficient of variation and a skill index. Moreover, there was some evidence of a dissociation between age and AD, such that healthy older participants showed disproportionate slowing after errors compared to middle-aged participants, but the AD individuals did not show a further increase in post-error slowing, but rather showed a small decrease compared to their age-matched controls.
In the following two sections, we discuss two aspects of the present data. First, we discuss the subjective/self-reported MW indices. We then turn to the more objective measures available from the SART. We believe these two dimensions ultimately converge on a useful framework.
Subjective/Self-reported mind-wandering
We formulated two competing hypotheses regarding mind-wandering in early AD. The cognitive resource account of MW (Smallwood & Schooler, 2006) predicts that AD individuals would report less MW because MW is a resource-dependent process and these individuals have depleted cognitive resources. A response style account would, however, predict that since early stage AD individuals are less conscientious than age-matched healthy controls (Duchek et al., 2007), they might show a less disciplined response style leading to more mind-wandering (see Jackson & Balota, 2012).
Our results support the cognitive resource hypothesis. We found not only an age-related decrease in tune out frequency in healthy participants, but also that AD individuals reported fewer tune outs and more on-task thought than did healthy age-matched controls, suggesting that early AD pathology affects mind-wandering rates above and beyond the effect of age. This could reflect the fact that these individuals have depleted cognitive resources and so need to allocate relatively more attentional resources to the task, and this could have led to more on-task thought reports. This is partly supported by the finding that AD individuals rated the task to be more difficult than both of the healthy groups. (However, it should also be noted that difficulty ratings were only very weakly related to frequency of tune outs, and were not related to on-task thought reports.)
Two further findings of the present study are also broadly consistent with the cognitive resource account. First, working memory capacity as indicated by reading span was found to decrease as a function of age and CDR status suggesting that these groups do indeed differ in the amount of cognitive resources they have at their disposal. Second, there was a positive relationship between MW and working memory capacity, suggesting that the more resources a person has the more likely they are to mind-wander during task performance. Alternatively, because this relationship only holds true for tune outs (i.e., MW with awareness) – this correlation could indicate that working memory capacity merely affects the ability to consciously report episodes of MW. Decline in working memory capacity, however, was only partially responsible for group differences in MW because differences between healthy controls and AD individuals were still present after controlling for working memory capacity. This suggests that some other factor, such as changes in metacognition (as discussed below) is also important in accounting for the AD-related decrease in self-reported MW.
Our results are inconsistent with the idea that episodes of MW reflect failures of executive control (McVay & Kane, 2009; 2012b) because both healthy and AD older adults would have been expected to report more off-task thoughts if this were the case. Furthermore, the non-negative association between MW and working memory capacity observed in our sample also speaks against this idea. An extension of this account is the “cognitive failure × current concerns” account (McVay & Kane, 2010; 2012b) which proposes that the occurrence of MW is not determined solely by the functioning of the executive control system but also by the number of competing personal goal-related thoughts a person has that might divert attention away from the primary task. Although this account can explain the typical pattern of age differences in MW comparing college students and older adults (McVay & Kane, 2010; Jackson & Balota, 2012), additional assumptions would have to be made to account for the findings of the present study. Specifically, it is unclear why two groups of community dwelling healthy older adults (62 years and 77 years old) differing in age by only 15 years would have different levels of current concerns. Moreover, one would also need to assume that the early stage AD individuals have fewer current concerns or concerns triggered by a laboratory setting than healthy adults of similar age. Given their diagnosis, one might expect the early stage AD individuals to have more current concerns when coming for cognitive testing in a laboratory setting. Although it is possible that current concerns contributed to the current results, further work would need to be conducted to demonstrate a clear link between current concerns and the reports of MW in these groups.
The findings of the present study are in line with previous research suggesting breakdowns in the default mode network in early stages of AD (Greicius et al., 2004; Lee et al., 2016; Lustig et al., 2003). This network is thought to play an important role in spontaneous cognition, including MW (Andrews-Hanna et al., 2010; Andrews-Hanna, Smallwood, & Spreng, 2014; Christoff et al., 2009, 2016; Durantin, Dehais, & Delorme, 2015; Mason et al., 2007; Stawarcyzk, Majerus, Maquet, & D’Argembeau, 2011). Specifically, it appears that the brain regions of the DMN overlap with those most vulnerable to deposition of the amyloid-β protein, an important pathological marker of AD (Mormino et al., 2011; Ouchi & Kikuchi, 2012; Sperling et al., 2009), which may appear even in preclinical AD groups (Sperling, Mormino, & Johnson, 2014). Therefore, it is possible that amyloid-β deposition disrupts DMN functioning, and this disruption directly affects the spontaneous generation of mental content, thus providing the neural basis for a decrease in MW frequency (e.g, Sheline et al., 2010). Although the present results are consistent with this perspective, it is important to note that the precise role of the DMN and its relation to MW in older adults remains unclear (Maillet & Schacter, 2016b). Specifically, DMN disruption might contribute to increased or decreased MW, changes in MW phenomenology (Andrews-Hanna, Smallwood, & Spreng, 2014), or perhaps even a change in an individual’s ability to report MW. Clearly, direct measures of DMN functional connectivity or DMN suppression during the SART in these groups would be necessary to directly examine this issue.
In accordance with previous studies (Duberstein et al., 2011; Duchek et al., 2007) we found that AD individuals were more neurotic and less conscientious than healthy controls. Contrary to expectations, however, conscientiousness was unrelated to self-reports of MW in the present sample. This personality variable did show correlations with some measures of SART performance: conscientious participants were faster, less variable in their responses, and showed overall better performance. These results suggest that more conscientious individuals do indeed adopt a more disciplined response tendency in the SART as hypothesized by Jackson and Balota (2012), but this does not affect how frequently they classify their thoughts as task-unrelated in self-report. As such, this cannot account for the decreased self-reported incidence of MW.
It is important to note that in this sample consisting largely of older individuals, participants rarely indicated experiencing that their mind was blank during the task (space out) or that they were unaware that their mind had wandered (zone out). Our results regarding self-reports of off-task thought only include frequency of tune outs, also known as mind-wandering with awareness. This raises the possibility that our findings merely reflect a decline in metacognitive awareness as a function of age and AD pathology, i.e., older adults and AD individuals might have difficulties monitoring their mental contents. Studies focusing on metacognition in AD outside the context of metamemory (Cosentino, 2014) are needed to address this possibility.
As a final note, interest ratings were found to be related to both on-task thought reports and tune outs. Those who found the task more interesting disengaged less frequently. This is in accordance with previous studies (Krawietz et al., 2012; Shake et al., 2015). Moreover, interest ratings were affected by both age and AD status – healthy middle-aged participants found the task the least interesting, while AD individuals found it more interesting than any other group. Controlling for interest, however, did not affect the age- and AD-effect in reported MW suggesting that the observed group differences are not simply attributable to greater interest in the task. This finding is important as it provides further support to the view that MW necessitates attentional resources, and that these resources modulate off-task thought, or perhaps metacognitive awareness of MW.
Behavioral performance on the SART
Although the self-reported indices of MW indicate that early stage AD appears to be a progression of healthy aging processes, this was not the case for all of the more objective measures. Reaction time to Go stimuli increased as a function of age in our sample, and there was further slowing in the early stage AD group above and beyond the effect of age. This, however, did not mean that the latter group adopted a more cautious response strategy: No-Go accuracy was the lowest in this group. Furthermore, the skill index (Seli, 2016) similarly showed both an age-related decline and a separate effect of AD pathology. In other words, skill diminished with age, and further diminished as a function of AD status. The general pattern of results is consistent with previous findings that suggest that attentional control tasks can be used to effectively distinguish between healthy older adults and older adults in the earliest stages of AD (Balota et al., 2010; Belleville, Bherer, Lepage, Chertkow, & Gauthier, 2008; Hutchison, Balota, & Duchek, 2010; Perry & Hodges, 1999; Spieler, Balota, & Faust, 1996).
We examined the two most commonly analyzed behavioral indices of MW in our sample: pre-error speeding, and post-error slowing. With respect to pre-error speeding, we found that participants were faster on trials preceding an error than on trials preceding a correctly withheld target trial. This has been proposed to reflect becoming disengaged from the task and responding in a mindless, automatic manner, leading to an error (Smallwood & Schooler, 2006). In the present study, no group differences were found in the magnitude of this pre-error speeding. These results are in line with Jackson and Balota’s (2012) finding that there were no age-related changes in pre-error speeding in healthy adults. Furthermore, the data also imply that AD pathology has no effect on pre-error speeding.
Post-error slowing showed a more complex pattern across groups. First, focusing on the two healthy groups, we replicated and extended the results of Jackson and Balota (2012) showing greater post-error slowing in a group of older old adults compared to a slightly younger older adult group. Post-error slowing was present in AD individuals as well, but its magnitude was not reliably different from the younger healthy group and was marginally smaller than the older adult group after processing speed was controlled. In short, instead of showing an increase in post-error slowing, these individuals produced a slight decrease compared to their age-matched controls. If post-error slowing does in fact reflect task re-engagement following periods of inattention (Cheyne et al., 2009; Jackson & Balota, 2012), it would seem this process might be deficient in AD older adults. This could be due to a number of reasons. For example, AD individuals may have trouble initiating the re-engagement of the task. In addition, because no feedback was given during the SART, it is possible that AD individuals are less likely to be aware of the errors and hence produce less post-error slowing. To our knowledge, error awareness in AD has not been investigated in a sustained attention paradigm thus far. In other paradigms, however, error awareness has been found to be both relatively intact (in lexical tasks; Ito & Kitagawa, 2005; Mathalon et al., 2003) and relatively impaired (in metamemory tasks, Cosentino, 2014) in AD individuals, and hence, there does not appear to be a clear consensus regarding predictions for the present paradigm. It is also possible that post-error slowing is a marker of a process other than task re-engagement. Notebaert et al. (2009) proposed that infrequent events such as errors lead to longer RTs because they capture attention and orient it away from the task. This re-orienting process may be deficient in the early AD group (Balota & Faust, 2001; Perry & Hodges, 1999).
We also investigated behavioral variability across groups, because variability has been proposed to be a marker of mind-wandering (Bastian & Sackur, 2013; Cheyne et al., 2009; Henríquez, Chica, Billeke, & Bartolomeo, 2016; Seli, Cheyne, & Smilek, 2013). Cheyne et al. (2009) suggested that RT variability is an indicator of “occurrent task inattention”, or brief attentional disengagements from the task at hand. In their model, these brief episodes of inattention are similar to tune outs in our study. Replicating previous studies not focusing on MW (e.g., Duchek et al., 2009; Hultsch, MacDonald, Hunter, Levy-Bencheton, & Strauss, 2000; Jackson, Balota, Duchek, & Head, 2012), we found that AD individuals produced a higher coefficient of variation (CoV) than did healthy controls. This would suggest that there is an increase in occurrent task inattention in the earliest stages of AD pathology. This, however, as reported above, was not reflected in higher rates of tune outs in these participants. In fact, the AD group reported tune outs with the lowest frequency. This could either reflect that self-reports on thought probes are not adequate reflections of the actual subjective experiences of early stage AD individuals, or that behavioral variability reflects other processes in addition to transient lapses in attention (e.g., changes in neural signal transmission; Duchek et al., 2009; Hultsch et al., 2000). The first alternative is closely related to metacognitive monitoring in AD mentioned above.
Summary
The present results extended past work showing decreased self-reported mind wandering in older adults, compared to younger adults. In addition, these results replicate the observation that while pre-error speeding does not show an age-related change, post-error slowing shows a disproportionate increase in older adults. Moreover, the older adults reported that they found the task more interesting compared to middle-aged adults, again replicating previous results. Importantly, these patterns were shown in two large cohorts of healthy community dwelling individuals, as opposed to typical aging studies comparing community dwelling older adults to college-aged samples, and hence, these age differences are not due to idiosyncratic characteristics of college-aged students. The self-reported mind wandering indices along with self-reported interest and difficulty changed further in early stage AD compared to age-matched older adults, suggesting AD is on a continuum with aging in these self-reports. However, there were some clear differences in the objective measures of SART performance. Specifically, we demonstrated an AD-specific increase in the CoV, and early stage AD individuals produced more comparable post-error slowing to the young old adults, in comparison to their age-matched older adults. We believe this latter effect, in conjunction with the self-reported increase in interest in the task, is most consistent with a decrease in self-monitoring in early stage AD that is specific to the AD pathophysiological cascade.
Figure 2.
Post-target standardized response latencies as a function of group and trial type. Error bars represent +/− 1 SE.
Public significance.
The present paper adds to the growing body of evidence that suggests that individuals in the early stages of Alzheimer’s disease show measurable changes in attention, a non-memory domain of cognition, by showing that these individuals report fewer episodes of inattention (mind-wandering) during a sustained attention task than healthy controls. Furthermore, our findings support the notion that self-reports of mind-wandering require cognitive resources, and are not simply a consequence of cognitive failures.
Acknowledgments
This study was funded by the following grants from the National Institute on Aging: P01AG03991 and P01AG026276.
Footnotes
We removed 4 additional participants whose data points lied outside the distribution of at least one of the main behavioral outcome variables (Go RT, No-Go RT, Pre-error RT, Pre-correct RT, Post-error RT, Post-correct RT) based on visual inspection of the histograms.
Because the group variable was also involved in a significant interaction, the main effect of group on RTs was further investigated in two separate between-subject ANOVAs for Go RT and No-Go RT. When Go RT was the dependent variable, there was a significant slowing in the AD group compared to the healthy older adult group (p < .05). When No-Go RT was the dependent variable, there was no significant difference between these two groups (p = .974).
The non-parametric tests also indicated a weakly significant difference between middle-aged adults and AD individuals in zone out frequency, but this effect is not interpreted further because it was not robust (p = .03). Associations between thought reports and behavioral performance (mean Go RT, No-Go accuracy, CV, skill index, pre-error RT z scores, and post-error RT z scores) were also investigated both in the whole sample and within each group. Some weak correlations were observed, all in the expected directions, however these results are not reported in detail because some thought report categories occurred with extremely low frequency thus distributions were heavily skewed. Furthermore, there was no clear age- or dementia status related change in the pattern of the relationships.
References
- Albert M, Moss MB, Blacker D, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology. 2007;21(2):158–169. doi: 10.1037/0894-4105.21.2.158. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology. 2010;104(1):322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews- Hanna JR, Smallwood J, Spreng RN. The default network and selfgenerated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316(1):29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Faust M. Attention in Dementia of the Alzheimer’s Type. In: Boller F, Cappa S, editors. Handbook of Neuropsychology. 2. Vol. 6. New York, NY: Elsevier Science; 2001. pp. 51–80. [Google Scholar]
- Balota DA, Tse CS, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer’s type in a healthy control sample: The power of errors in Stroop color naming. Psychology and Aging. 2010;25(1):208–218. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian M, Sackur J. Mind-wandering at the fingertips: automatic parsing of subjective states based on response time variability. Frontiers in Psychology. 2013;4:573. doi: 10.3389/fpsyg.2013.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Bherer L, Lepage É, Chertkow H, Gauthier S. Task switching capacities in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychologia. 2008;46(8):2225–2233. doi: 10.1016/j.neuropsychologia.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The serendipitous discovery of the brain’s default network. Neuroimage. 2012;62(2):1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12(5):543–550. [Google Scholar]
- Cheyne JA, Carriere JS, Solman GJ, Smilek D. Challenge and error: Critical events and attention-related errors. Cognition. 2011;121(3):437–446. doi: 10.1016/j.cognition.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Cheyne JA, Solman GJ, Carriere JS, Smilek D. Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition. 2009;111(1):98–113. doi: 10.1016/j.cognition.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences. 2009;106(21):8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Irving ZC, Fox KC, Spreng RN, Andrews-Hanna JR. Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews Neuroscience. 2016;17(11):718–731. doi: 10.1038/nrn.2016.113. [DOI] [PubMed] [Google Scholar]
- Cosentino SA. Metacognition in Alzheimer’s disease. In: Fleming SM, Frith CD, editors. The Cognitive Neuroscience of Metacognition. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. pp. 389–407. [Google Scholar]
- Costa PT, McCrae RR. Revised NEO personality inventory (NEO PI-R) and NEO five-factor inventory (NEO FFI): Professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Craik FI. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Amsterdam, North-Holland: Elsevier Science Publishing Company; 1986. pp. 409–422. [Google Scholar]
- Cunningham S, Scerbo MW, Freeman FG. The electrocortical correlates of daydreaming during vigilance tasks. Journal of Mental Imagery. 2000;24(1–2):61–72. [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19(4):450–466. [Google Scholar]
- Duberstein PR, Chapman BP, Tindle HA, Sink KM, Bamonti P, Robbins J, Jerant AF, Franks P. Personality and risk for Alzheimer’s disease in adults 72 years of age and older: a 6-year follow-up. Psychology and Aging. 2011;26(2):351–362. doi: 10.1037/a0021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Storandt M, Larsen R. The power of personality in discriminating between healthy aging and early-stage Alzheimer’s disease. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2007;62(6):353–361. doi: 10.1093/geronb/62.6.p353. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Tse CS, Holtzman DM, Fagan AM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology. 2009;23(6):746–758. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durantin G, Dehais F, Delorme A. Characterization of mind wandering using fNIRS. Frontiers in Systems Neuroscience. 2015;9:45. doi: 10.3389/fnsys.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust ME, Balota DA. Inhibition, Facilitation, and Attention Control in Dementia of the Alzheimer Type: The role of unifying principles in cognitive theory development. In: Gorfein DS, MacLeod CM, editors. Inhibition in cognition. Washington, DC: American Psychological Association; 2007. pp. 213–238. [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: implications for group differences in response latency. Psychological Bulletin. 1999;125(6):777–799. doi: 10.1037/0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Frank DJ, Nara B, Zavagnin M, Touron DR, Kane MJ. Validating older adults’ reports of less mind-wandering: An examination of eye movements and dispositional influences. Psychology and Aging. 2015;30(2):266–278. doi: 10.1037/pag0000031. [DOI] [PubMed] [Google Scholar]
- Giambra LM. Task-unrelated-thought frequency as a function of age: A laboratory study. Psychology and Aging. 1989;4(2):136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- Giambra LM. The influence of aging on spontaneous shifts of attention from external stimuli to the contents of consciousness. Experimental Gerontology. 1993;28(4):485–492. doi: 10.1016/0531-5565(93)90073-m. [DOI] [PubMed] [Google Scholar]
- Giambra L. Daydreaming characteristics across the life-span: Age differences and seven to twenty year longitudinal changes. In: Kunzendorf RG, Wallace B, editors. Individual differences in conscious experience. Amsterdam, Netherlands: John Benjamins Publishing Company; 2000. pp. 147–206. [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henríquez RA, Chica AB, Billeke P, Bartolomeo P. Fluctuating minds: spontaneous psychophysical variability during mind-wandering. PLoS One. 2016;11(2):e0147174. doi: 10.1371/journal.pone.0147174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in older adults: comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14(4):588–598. doi: 10.1037//0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Hutchison KA, Balota DA, Duchek JM. The utility of Stroop task switching as a marker for early-stage Alzheimer’s disease. Psychology and Aging. 2010;25(3):545–559. doi: 10.1037/a0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J, Kitagawa J. Error processing in patients with Alzheimer’s disease. Pathophysiology. 2005;12(2):97–101. doi: 10.1016/j.pathophys.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Jackson JD, Balota DA. Mind-wandering in younger and older adults: converging evidence from the Sustained Attention to Response Task and reading for comprehension. Psychology and Aging. 2012;27(1):106–119. doi: 10.1037/a0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JD, Balota DA, Duchek JM, Head D. White matter integrity and reaction time intraindividual variability in healthy aging and early-stage Alzheimer disease. Neuropsychologia. 2012;50(3):357–366. doi: 10.1016/j.neuropsychologia.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JD, Weinstein Y, Balota DA. Can mind-wandering be timeless? Atemporal focus and aging in mind-wandering paradigms. Frontiers in Psychology. 2013;4:742. doi: 10.3389/fpsyg.2013.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18(7):614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Kirschner A, Kam JWY, Handy TC, Ward LM. Differential synchronization in default and task-specific networks of the human brain. Frontiers in Human Neuroscience. 2012;6:139. doi: 10.3389/fnhum.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawietz SA, Tamplin AK, Radvansky GA. Aging and mind wandering during text comprehension. Psychology and Aging. 2012;27(4):951–958. doi: 10.1037/a0028831. [DOI] [PubMed] [Google Scholar]
- Lee ES, Yoo K, Lee YB, Chung J, Lim JE, Yoon B, Jeong Y. Default Mode Network Functional Connectivity in Early and Late Mild Cognitive Impairment: Results From the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer Disease and Associated Disorders. 2016;30(4):289–296. doi: 10.1097/WAD.0000000000000143. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Schacter DL. From mind wandering to involuntary retrieval: Age-related differences in spontaneous cognitive processes. Neuropsychologia. 2016a;80:142–156. doi: 10.1016/j.neuropsychologia.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Schacter DL. Default network and aging: Beyond the task-negative perspective. Trends in Cognitive Sciences. 2016b;20(9):646–648. doi: 10.1016/j.tics.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Bennett A, Askari N, Gray EM, Rosenbloom MJ, Ford JM. Response-monitoring dysfunction in aging and Alzheimer’s disease: an event-related potential study. Neurobiology of Aging. 2003;24(5):675–685. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:934–939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(1):196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008) Psychological Bulletin. 2010;136(2):188–197. doi: 10.1037/a0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Drifting from slow to “d’oh!”: Working memory capacity and mind wandering predict extreme reaction times and executive control errors. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012a;38(3):525–549. doi: 10.1037/a0025896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Why does working memory capacity predict variation in reading comprehension? On the influence of mind wandering and executive attention. Journal of Experimental Psychology: General. 2012b;141(2):302–320. doi: 10.1037/a0025250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ, Kwapil TR. Tracking the train of thought from the laboratory into everyday life: An experience-sampling study of mind wandering across controlled and ecological contexts. Psychonomic Bulletin & Review. 2009;16(5):857–863. doi: 10.3758/PBR.16.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Meier ME, Touron DR, Kane MJ. Aging ebbs the flow of thought: Adult age differences in mind wandering, executive control, and self-evaluation. Acta Psychologica. 2013;142(1):136–147. doi: 10.1016/j.actpsy.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cerebral Cortex. 2011;21(10):2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Notebaert W, Houtman F, Van Opstal F, Gevers W, Fias W, Verguts T. Post-error slowing: an orienting account. Cognition. 2009;111(2):275–279. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Kikuchi M. A review of the default mode network in aging and dementia based on molecular imaging. Reviews in the Neurosciences. 2012;23(3):263–268. doi: 10.1515/revneuro-2012-0029. [DOI] [PubMed] [Google Scholar]
- Parks CW, Klinger E, Perlmutter M. Dimensions of thought as a function of age, gender and task difficulty. Imagination, Cognition and Personality. 1988;8(1):49–62. [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122(3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35(6):747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Reichle ED, Schooler JW. Lost in the sauce: The effects of alcohol on mind wandering. Psychological Science. 2009;20(6):747–752. doi: 10.1111/j.1467-9280.2009.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler JW, Reichle ED, Halpern DV. Zoning-out while reading: Evidence for dissociations between experience and meta-consciousness. In: Levin DT, editor. Thinking and seeing: Visual metacognition in adults and children. Cambridge, MA: MIT Press; 2004. pp. 203–226. [Google Scholar]
- Schooler JW, Smallwood J, Christoff K, Handy TC, Reichle ED, Sayette MA. Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences. 2011;15(7):319–326. doi: 10.1016/j.tics.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Seli P. The Attention-Lapse and Motor Decoupling accounts of SART performance are not mutually exclusive. Consciousness and Cognition. 2016;41:189–198. doi: 10.1016/j.concog.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, Smilek D. Wandering minds and wavering rhythms: Linking mind wandering and behavioral variability. Journal of Experimental Psychology: Human Perception and Performance. 2013;39(1):1–5. doi: 10.1037/a0030954. [DOI] [PubMed] [Google Scholar]
- Seli P, Jonker TR, Solman GJ, Cheyne JA, Smilek D. A methodological note on evaluating performance in a Sustained-Attention-To-Response Task. Behavior Research Methods. 2013;45(2):355–363. doi: 10.3758/s13428-012-0266-1. [DOI] [PubMed] [Google Scholar]
- Shake MC, Shulley LJ, Soto-Freita AM. Effects of individual differences and situational features on age differences in mindless reading. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2015;71(5):808–820. doi: 10.1093/geronb/gbv012. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological Psychiatry. 2010;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Singer J, McCraven V. Some characteristics of adult daydreaming. Journal of Psychology. 1961;51:151–164. [Google Scholar]
- Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience. 2008;20(3):458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Davies JB, Heim D, Finnigan F, Sudberry M, O’Connor R, Obonsawin M. Subjective experience and the attentional lapse: Task engagement and disengagement during sustained attention. Consciousness and Cognition. 2004;13(4):657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin & Review. 2007;14(3):527–533. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. When attention matters: The curious incident of the wandering mind. Memory & Cognition. 2008;36(6):1144–1150. doi: 10.3758/MC.36.6.1144. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychological Bulletin. 2006;132(6):946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The science of mind wandering: empirically navigating the stream of consciousness. Annual Review of Psychology. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84(3):608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22(2):461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Staub B, Doignon-Camus N, Bacon E, Bonnefond A. Investigating sustained attention ability in the elderly by using two different approaches: inhibiting ongoing behavior versus responding on rare occasions. Acta Psychologica. 2014;146:51–57. doi: 10.1016/j.actpsy.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica. 2011;136(3):370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PloS One. 2011;6(2):e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs revised MCI and in pre-MCI. Neurology. 2006;67(3):467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Zavagnin M, Borella E, De Beni R. When the mind wanders: Age-related differences between young and older adults. Acta Psychologica. 2014;145:54–64. doi: 10.1016/j.actpsy.2013.10.016. [DOI] [PubMed] [Google Scholar]