Abstract
Background
Among intensive care unit (ICU) patients with acute respiratory distress syndrome (ARDS), apart from acute cor pulmonale (ACP), the frequency and prognostic impact of basic critical care echocardiography (BCCE) abnormalities are not well defined.
Methods
Observational study of patients with ARDS, admitted from September 2012 to May 2014, who underwent BCCE within 48 h of admission to a 20-bed medical ICU. We examined the association of two major BCCE-detected abnormalities (left ventricular ejection fraction < 40% and severe ACP) with ICU/hospital mortality and ICU/hospital length of stay. Multivariable models adjusted for age and illness severity.
Results
Of 234 patients with ARDS (age 62.3 ± 14.3 years; 88/37.6% female; APACHE II 26.8 ± 8.3; 26.5% ICU mortality; 32.1% hospital mortality), 94 (40.2%) had at least one major BCCE-detected abnormality. The more common major BCCE abnormality found was severe ACP (28.2%), followed by left ventricular ejection fraction < 40% (16.2%). On multivariate analysis, only severe ACP remained significantly associated with ICU/hospital mortality. Hospital mortality for mild, moderate and severe ARDS was 17.0, 27.9 and 50.0%, respectively (without severe ACP), and was 29.2, 48.3 and 53.8%, respectively (with severe ACP).
Conclusions
BCCE abnormalities were common, but only severe ACP had prognostic significance in ARDS, identifying patients who are at increased risk of ICU and hospital mortality. The presence of severe ACP appears to upstage ARDS severity by one level.
Keywords: Echocardiography; Intensive care units; Respiratory distress syndrome, adult; Cor pulmonale; Ventricular dysfunction, left
Background
Acute respiratory distress syndrome (ARDS) is a common critical illness with high mortality [1]. Patients may develop cardiac complications as the result of severe illness or as a side effect of treatment. In particular, patients with ARDS may develop right ventricular overload and acute cor pulmonale (ACP) [2–4]. The pathophysiology of ACP is complex and is related to pulmonary vasoconstriction, permissive hypercapnia, intrapulmonary microthrombi and positive pressure mechanical ventilation [4–6].
The spread of basic critical care echocardiography (BCCE) technology and expertise will allow intensive care unit (ICU) physicians to incorporate BCCE into routine clinical practice. For instance, BCCE has been recognized as a useful tool for hemodynamic optimization of patients known to have shock [7, 8]. BCCE can also be used to detect two major echocardiographic abnormalities: left ventricular ejection fraction and severe ACP [9].
Apart from ACP, there is uncertainty over the frequency and prognostic significance of the above-mentioned two major BCCE abnormalities in newly admitted ICU patients with ARDS. Knowing this information would be useful for delineating the potential role of BCCE screening for such critically ill patients, which is currently unclear [10]. We therefore aimed to investigate the frequency of BCCE-detected abnormalities in patients with ARDS and to elucidate any associations with ICU and hospital mortality, or with increased ICU or hospital length of stay.
Methods
Participants and setting
We conducted a prospective observational study of patients with ARDS admitted to our 20-bed medical ICU from September 2012 to May 2014, who underwent BCCE within 48 h of admission. Due to manpower constraints, BCCE was routinely scheduled on three weekdays (Monday, Wednesday and Friday) and was done on an ad hoc basis over weekends and public holidays for newly admitted patients. BCCE was not done if patients discharged or died before it could be done. Three patients, who had surgical dressings over the chest/abdomen precluding satisfactory transthoracic echocardiographic windows, were excluded. No other exclusion criteria were used. Only the first ICU admission for each patient was analysed. The presence and severity of ARDS were determined on the day of BCCE.
Our Ethics Review Board (National Healthcare Group Domain-Specific Review Board) permitted waiver of informed consent (DSRB B/2013/00132). Although we reported some of our data in a prior study on BCCE training [9], our current study has different aims, the number of ARDS patients has been doubled and we have collected new information pertaining to ARDS.
Scanning procedure and clinical care
BCCE was only performed by competent attending physicians or fellows, or by fellows under supervision, using the Sparq Ultrasound System (Philips Healthcare, Andover, MA) equipped with a 2–4 MHz broadband sector, phased array transducer. Physicians were deemed to be competent if they had at least 1 year of daily experience with BCCE performance and interpretation. At least seven standard views (acoustic windows) were obtained and recorded for each BCCE scan: parasternal long axis, parasternal short axis, apical four-chamber (three views), subcostal and inferior vena cava (IVC) [9]. For this study, we considered two major abnormalities because these are reliably detected by bedside echocardiography: visually estimated left ventricular ejection fraction < 40% and severe ACP (right ventricular dilatation with the right-to-left ventricular size (area) ratio ≥ 1 in end diastole at the papillary muscle level and interventricular septal straightening/paradoxical motion using the parasternal short axis view) [9, 11, 12]. The presence of ACP was determined by visually examining the relative sizes of the right and left ventricles in both the parasternal short axis views and the apical four-chamber views. We chose the parasternal short axis view as the main view to assess the relative sizes of the right and left ventricles and to assess for septal straightening/paradoxical motion (see Fig. 1 for an example), as this view had a fixed landmark (papillary muscles) and was not prone to foreshortening or rotational error. Since we relied on a ratio of 1 between the right and left ventricle sizes, we also found that visual comparison was rapid and accurate without routine manual tracing of the endocardial borders. We used the apical four-chamber as a secondary safeguard against false ACP determination, which in our experience did not occur. Conversely, if we had used the apical four-chamber view as the main view, potential foreshortening or rotational error would lead to under-recognition of RV dilatation and ACP. We determined that the cor pulmonale was acute, rather than chronic, if there was no significant right ventricular dysfunction noted previously, either clinically or from prior echocardiography.
Fig. 1.
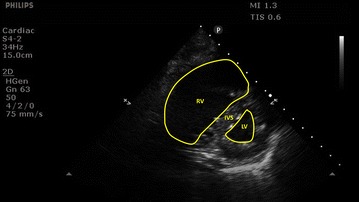
An example of acute cor pulmonale. 82-year-old woman with myelodysplastic syndrome was intubated for severe community-acquired pneumonia. She had no known cardiac problems or chronic lung disease. On admission to the intensive care unit, basic echocardiography was done. The parasternal short axis view showed a dilated right ventricle (RV) in end diastole at the papillary muscle level and interventricular septum (IVS) straightening, indicating acute cor pulmonale. The RV and left ventricle (LV) areas may be determined via endocardial tracing as shown, though in many cases, an RV/LV ratio of ≥ 1 can be determined visually without routine manual tracing
Usage of BCCE findings and clinical care were left to the discretion of the attending physicians. During the time period of the study, we did not enforce any protocol with regard to specific BCCE abnormalities or mandate repeat scanning. For hemodynamic management, our ICU relied on arterial line blood pressure [13], with the option of FloTrac/Vigileo (Edwards Lifesciences, Irvine, CA) continuous arterial pressure waveform-based cardiac output measurements and echocardiography. Noradrenaline was the preferred vasopressor, particularly for septic shock [14]. For ventilator management, patients received low tidal volume ventilation with minimal analgesia and sedation, but not high-frequency oscillatory ventilation [15] or nitric oxide [16]. None of the patients received prone positioning as this was not usual practice for our ICU during the study period. Sepsis was treated with early, broad-spectrum antibiotics and source control.
Data collection
Clinical data were extracted from the ICU computerized database and medical records. The latest arterial blood gas measurement, which was taken on the day of the BCCE scan, was used to compute the arterial oxygen partial pressure (in mmHg) to inspired oxygen fraction (PF ratio). Respiratory parameters extracted from the database included tidal volume, respiratory rate and positive end-expiratory pressure at the time of BCCE. We did not routinely perform inspiratory pause manoeuvres at the time of BCCE. These manoeuvres were nonetheless done routinely for mechanically ventilated patients every morning by our respiratory therapists, and we recorded plateau pressures and respiratory compliance readings taken on the morning of the BCCE scan.
ARDS was defined using the Berlin Definition [17]. To diagnose ARDS, patients needed a PF ratio < 300 mmHg, a positive end-expiratory pressure ≥ 5 cm H2O (may be non-invasively delivered for mild ARDS), no predominant cardiac failure or fluid overload and bilateral infiltrates on chest radiography. These features must also develop within 1 week of a known clinical insult or new or worsening respiratory symptoms. The severity of ARDS was classified as mild (PF ratio 201–300 mmHg), moderate (PF ratio 101–200 mmHg) and severe (PF ratio 100 mmHg or less).
Statistical analysis
Univariate comparisons of proportions, means and medians were, respectively, done using Fisher exact, Student t and Wilcoxon rank-sum tests. Confidence intervals of binomial probability distributions were computed using the Clopper–Pearson (exact) method. We examined the association of major BCCE-detected abnormalities with ICU/hospital mortality and ICU/hospital length of stay. Logistic regression was used to analyse mortality as an outcome. Linear regression was used to analyse length of stay (log-transformed to achieve normality) as an outcome. Both the logistic regression and linear regression models corrected for age, and Acute Physiology and Chronic Health Evaluation (APACHE) II score. Statistical significance was taken as P < 0.05.
Results
During the study period, 1218 patients (1371 admissions; age 61.8 ± 16.5 years; 492/40.4% female; APACHE II 22.7 ± 9.0) were treated in our ICU. We performed BCCE for 651 patients (709 admissions; age 61.0 ± 16.6 years; 251/38.6% female; APACHE II 24.0 ± 8.2). Although we did not collect information on the presence or absence of ARDS for patients who did not receive BCCE, these patients did not differ from the whole ICU population in terms of age (P = 0.973), gender (P = 0.448) and APACHE II score (P = 0.915).
Among patients who received BCCE, 234 patients fulfilled the Berlin Definition of ARDS (age 62.3 ± 14.3 years; 88/37.6% female; APACHE II 26.8 ± 8.3) (Table 1). Ninety-four patients (40.2%) had at least one major BCCE-detected abnormality. The more common major BCCE abnormality found was severe ACP (28.2%), followed by left ventricular ejection fraction < 40% (16.2%). Among our 66 patients with ACP, no patient had any clinical cor pulmonale at baseline. Additionally, of 42 (63.6% of 66) patients who had prior transthoracic echocardiography, no patient had moderate or severe right ventricular dysfunction noted, and only 3 (4.6% of 66) patients had mild right ventricular dysfunction noted. Hospital mortality was 20.5% (83 patients, 95% CI 12.4–30.8%) for mild ARDS, 33.3% (108 patients, 95% CI 24.6–43.1%) for moderate ARDS and 51.2% (43 patients, 95% CI 35.5–66.7%) for severe ARDS (Table 2).
Table 1.
Patient characteristics and outcomes
| Patient characteristic or outcome | All patients with ARDS (N = 234) | Patients with ARDS, without severe ACP (N = 168) | Patients with ARDS, with severe ACP (N = 66) | P value |
|---|---|---|---|---|
| Age (years) (mean ± SD) | 62.3 ± 14.3 | 62.0 ± 14.7 | 64.8 ± 12.9 | 0.180 |
| Female sex (%) | 88 (37.6) | 65 (38.7) | 23 (34.9) | 0.654 |
| APACHE II score (mean ± SD) | 26.8 ± 8.3 | 26.7 ± 8.1 | 27.3 ± 8.7 | 0.615 |
| Arterial blood gas measurement | ||||
| PF ratio (mmHg) (mean ± SD) | 171 ± 67 | 172 ± 69 | 169 ± 63 | 0.801 |
| pH (mean ± SD) | 7.33 ± 0.12 | 7.34 ± 0.11 | 7.31 ± 0.13 | 0.065 |
| PaCO2 (mmHg) (mean ± SD) | 43 ± 14 | 42 ± 13 | 47 ± 16 | 0.001 |
| ARDSa (%) | ||||
| Mild | 83 (35.5) | 59 (35.1) | 24 (36.4) | 0.892 |
| Moderate | 108 (46.2) | 79 (47.0) | 29 (43.9) | |
| Severe | 43 (18.4) | 30 (17.9) | 13 (19.7) | |
| Primary diagnosis (%) | ||||
| Pneumonia | 208 (88.9) | 151 (89.9) | 57 (86.4) | 0.489 |
| Non-pneumonia sepsis | 26 (11.1) | 17 (10.1) | 9 (13.6) | |
| Comorbidities (%) | ||||
| Diabetes mellitus | 81 (34.6) | 61 (36.3) | 20 (30.3) | 0.446 |
| Hypertension | 115 (49.2) | 86 (51.2) | 29 (43.9) | 0.384 |
| Ischaemic heart disease | 55 (23.5) | 43 (25.6) | 12 (18.2) | 0.304 |
| Chronic heart failure | 9 (3.9) | 7 (4.2) | 2 (3.0) | 1.000 |
| Asthma | 14 (6.0) | 10 (6.0) | 4 (6.1) | 1.000 |
| COPD | 17 (7.3) | 13 (7.7) | 4 (6.1) | 0.785 |
| Bronchiectasis | 10 (4.3) | 7 (4.2) | 3 (4.6) | 1.000 |
| Chronic renal failure | 38 (16.2) | 27 (16.1) | 11 (16.7) | 1.000 |
| Chronic liver disease | 10 (4.3) | 8 (4.8) | 2 (3.0) | 0.729 |
| Stroke | 16 (6.8) | 13 (7.7) | 3 (4.6) | 0.566 |
| Cancer | 39 (16.7) | 31 (18.5) | 8 (12.1) | 0.330 |
| Actual body weight (kg) (mean ± SD) | 63.3 ± 17.2 | 63.4 ± 16.6 | 62.9 ± 18.6 | 0.840 |
| Ventilation modes (%) | ||||
| Nil ventilation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.942 |
| CPAP | 17 (7.3) | 13 (7.7) | 4 (6.1) | |
| NIV | 10 (4.3) | 7 (4.2) | 3 (4.6) | |
| Invasive | 207 (88.5) | 148 (88.1) | 59 (89.4) | |
| Respiratory parameters at time of BCCE | ||||
| Respiratory rate (breaths/min) (mean ± SD) | 24 ± 3 | 24 ± 4 | 24 ± 2 | 0.274 |
| Tidal volume (ml) (mean ± SD) | 408 ± 113 | 409 ± 113 | 407 ± 112 | 0.931 |
| Tidal volume (ml/kg IBW) (mean ± SD) | 7 ± 2 | 7 ± 2 | 7 ± 3 | 0.276 |
| PEEP (cm H2O) (mean ± SD) | 7 ± 3 | 6 ± 3 | 7 ± 3 | 0.089 |
| Plateau pressureb (cm H2O) (mean ± SD) | 21 ± 3 | 21 ± 2 | 21 ± 5 | 0.507 |
| Complianceb (ml/cm H2O) (mean ± SD) | 31 ± 14 | 29 ± 12 | 34 ± 18 | 0.047 |
| On vasoactive agents (%) | ||||
| Any agentc | 77 (32.9) | 55 (32.7) | 22 (33.3) | 1.000 |
| Dopamine | 4 (1.7) | 2 (1.2) | 2 (3.0) | 0.316 |
| Noradrenaline | 73 (31.2) | 52 (31.0) | 21 (31.8) | 1.000 |
| Dobutamine | 2 (0.9) | 2 (1.2) | 0 (0.0) | 1.000 |
| Vasopressin | 1 (0.4) | 1 (0.6) | 0 (0.0) | 1.000 |
| BCCE-detected major abnormalities (%) | ||||
| Left ventricular ejection fraction < 40% | 38 (16.2) | 28 (16.7) | 10 (15.2) | 0.846 |
| Severe acute cor pulmonale | 66 (28.2) | 0 (0.0) | 66 (100.0) | < 0.001 |
| Any major abnormalities | 94 (40.2) | 28 (16.7) | 66 (100.0) | < 0.001 |
| LOS, ICU (days), median (IQR) | 7 (4–12) | 7 (4–12) | 7 (3–13) | 0.931 |
| LOS, hospital (days), median (IQR) | 17.5 (9–28) | 18 (9–26) | 17 (8–31) | 0.837 |
| Mortality, ICU (%) | 62 (26.5) | 37 (22.0) | 25 (37.9) | 0.021 |
| Mortality, hospital (%) | 75 (32.1) | 47 (28.0) | 28 (42.4) | 0.043 |
ACP Acute cor pulmonale (severe ACP is defined as right ventricular dilatation with the right-to-left ventricular size ratio ≥ 1 in end diastole at the papillary muscle level and interventricular septal straightening/paradoxical motion), APACHE II Acute Physiology and Chronic Health Evaluation II, ARDS acute respiratory distress syndrome, BCCE basic critical care echocardiography, COPD chronic obstructive pulmonary disease, CPAP continuous positive airway pressure, IBW ideal body weight. For males, IBW = 50 + 2.3 kg for each increment of 2.54 cm (1 inch) in length over 152.4 cm (5 feet). For females, IBW = 45.5 + 2.3 kg for each increment of 2.54 cm (1 inch) in length over 152.4 cm (5 feet), ICU intensive care unit, IQR interquartile range, LOS length of stay, PEEP positive end-expiratory pressure, SD standard deviation, NIV non-invasive ventilation
aARDS severity according to the Berlin Definition: mild (PF ratio 201–300 mmHg), moderate (PF ratio 101–200 mmHg) and severe (PF ratio 100 mmHg or less), where PF ratio is the ratio of arterial oxygen partial pressure (mmHg) to inspired oxygen fraction
bData only for the intubated patients (N = 207)
cPatients could be on more than one vasoactive agent
Table 2.
Hospital mortality of patients with acute respiratory distress syndrome, with or without severe acute cor pulmonale
| Hospital mortality | All ARDS patients (N = 234) | ARDS patients without severe ACP (N = 168) | ARDS patients with severe ACP (N = 66) | P valueb |
|---|---|---|---|---|
| Severity of ARDSa (%, CI) | ||||
| Mild | 17/83 (20.5, 12.4–30.8) | 10/59 (17.0, 8.4–29.0) | 7/24 (29.2, 12.6–51.1) | 0.239 |
| Moderate | 36/108 (33.3, 24.6–43.1) | 22/79 (27.9, 18.3–39.1) | 14/29 (48.3, 29.4–67.5) | 0.065 |
| Severe | 22/43 (51.2, 35.5–66.7) | 15/30 (50.0, 31.3–68.7) | 7/13 (53.8, 25.1–80.8) | 1.000 |
| Overall cohort (%, CI) | 75/234 (32.1, 26.1–38.4) | 47/168 (28.0, 21.3–35.4) | 28/66 (42.4, 30.3–55.2) | 0.043* |
ACP Acute cor pulmonale (severe ACP is defined as right ventricular dilatation with the right-to-left ventricular size ratio ≥ 1 in end diastole at the papillary muscle level and interventricular septal straightening/paradoxical motion), ARDS acute respiratory distress syndrome, CI 95% confidence interval
*P < 0.05
aARDS severity according to the Berlin Definition: mild (PF ratio 201–300 mmHg), moderate (PF ratio 101–200 mmHg) and severe (PF ratio 100 mmHg or less), where PF ratio is the ratio of arterial oxygen partial pressure (mmHg) to inspired oxygen fraction
bComputed for the mortality difference between patients with and without severe acute cor pulmonale, using the Fisher exact test
On multivariate analysis, among the major BCCE abnormalities, only severe ACP was associated with ICU and hospital mortality (Table 3). No associations between major BCCE abnormalities and ICU/hospital length of stay existed (Table 4). Hospital mortality for mild, moderate and severe ARDS was 17.0, 27.9 and 50.0%, respectively (without severe ACP), and was 29.2, 48.3 and 53.8%, respectively (with severe ACP) (Table 2).
Table 3.
Association of basic critical care echocardiography screening-derived abnormalities with mortality in patients with acute respiratory distress syndrome
| Major BCCE-detected abnormalities | ICU mortality | Hospital mortality | ||
|---|---|---|---|---|
| Univariate OR (95% CI)a | Multivariate OR (95% CI)b | Univariate OR (95% CI)a | Multivariate OR (95% CI)b | |
| Screened patients with acute respiratory distress syndrome (Berlin Definition) | ||||
| Left ventricular ejection fraction < 40% | 2.37 (1.15–4.89)* | 2.10 (0.99–4.43) | 2.19 (1.08–4.45)* | 2.00 (0.95–4.18) |
| Severe acute cor pulmonale | 2.16 (1.17–4.00)* | 2.14 (1.13–4.04)* | 1.90 (1.05–3.43)* | 1.89 (1.02–3.50)* |
BCCE Basic critical care echocardiography, CI confidence interval, ICU intensive care unit, OR odds ratio
*P < 0.05
aOdds ratio (with 95% confidence interval) derived using logistic regression on mortality, unadjusted
bOdds ratio (with 95% confidence interval) derived using multiple logistic regression on mortality, adjusted for age and Acute Physiology and Chronic Health Evaluation II score
Table 4.
Association of basic critical care echocardiography screening-derived abnormalities with log(length of stay) in patients with acute respiratory distress syndrome
| Major BCCE-detected abnormalities | Log (length of stay, ICU) | Log (length of stay, hospital) | ||
|---|---|---|---|---|
| Univariate ratio (95% CI)a | Multivariate ratio (95% CI)b | Univariate ratio (95% CI)a | Multivariate ratio (95% CI)b | |
| Screened patients with acute respiratory distress syndrome (Berlin Definition) | ||||
| Left ventricular ejection fraction < 40% | 1.00 (0.75–1.33) | 1.00 (0.74–1.34) | 0.84 (0.62–1.15) | 0.86 (0.63–1.17) |
| Severe acute cor pulmonale | 1.07 (0.84–1.36) | 1.10 (0.87–1.39) | 1.04 (0.81–1.34) | 1.06 (0.82–1.37) |
BCCE Basic critical care echocardiography, CI Confidence interval, ICU Intensive care unit
aExponentiated coefficient (with 95% confidence interval) derived using linear regression on the log-transformed LOS, unadjusted
bExponentiated coefficient (with 95% confidence interval) derived using multiple linear regression on the log-transformed LOS, adjusted for age and Acute Physiology and Chronic Health Evaluation II score
Discussion
The main findings of our study are as follows: firstly, BCCE abnormalities in ARDS patients were common, affecting 40% of the patients. Secondly, the presence of severe ACP—but not moderate/severe left ventricular dysfunction—within 48 h of ICU admission identified patients who were at increased risk of ICU and hospital mortality. Thirdly, both the BCCE-detected major abnormalities were not associated with ICU or hospital length of stay. Finally, the presence of severe ACP appears to upstage ARDS severity by one level.
Our study provided new information on the relative frequency of two major BCCE abnormalities in ARDS patients. We found that the more common abnormality was severe ACP, at 28.2%, which was around the same frequency demonstrated in another smaller study [18] and in a separate study focusing on severe H1N1 infection [19]. Nonetheless, this frequency was higher than the prevalence of severe ACP of 7% found in a prior multicentre study by Mekontso-Dessap and colleagues [3], which could be due to our non-adoption of strategies such as prone positioning to off-load the right ventricle and a higher proportion (89 vs. 40%) of pneumonia (pneumonia being a risk factor for ACP) in our cohort [5]. Across the ARDS severity gradient, ACP was fairly consistently found in 28.9, 26.9 and 30.2% of mild, moderate and severe ARDS cases. This would imply that, in our setting of high pneumonia prevalence, there was little interaction between ACP and ARDS severity. It is possible that ACP may be more a consequence of treatment strategies than disease manifestation, which would then make ACP potentially modifiable. Interestingly, among patients with ARDS, patients with ACP had slightly better respiratory system compliance compared to patients without ACP. This could reflect the lung recruitment effect of slightly higher positive end-expiratory pressure applied in cases of ACP.
Moderate/severe left ventricular dysfunction was less common in our study population, occurring in 16.2% of patients. Although previously published ARDS-specific data are not available, the frequency of left ventricular dysfunction in our cohort is consistent with prior data derived from patients with septic shock [20]. Similarly, our finding that left ventricular dysfunction had no association with mortality is also consistent with the lack of association in septic patients [21–23]. Given that we saw no increased mortality even though we only used inotropic medications very sparingly, our findings do not support the need to treat isolated left ventricular dysfunction in ARDS.
In contrast to left ventricular dysfunction, we found the presence of severe ACP to be particularly important for predicting mortality [3, 4, 11, 12]. Our definition of severe ACP involved a right-to-left ventricular size ratio ≥ 1 on transthoracic echocardiography, which corresponds to the definition of severe ACP on transesophageal echocardiography in a recent study [3]. The latter study also found that less severe ACP (i.e. right-to-left ventricular size ratio > 0.6 and < 1) was conversely not associated with mortality. The increased mortality engendered by severe ACP may be due to an increased incidence of circulatory failure in ARDS patients [4]. In our experience, such patients are harmed by fluid administration and often require moderate-to-high doses of noradrenaline support [5]. Separately, the absence of any relationship between BCCE findings and ICU/hospital length of stay is in line with prior data for ACP [4] and with our earlier study [9], which implies that length of stay may be more influenced by non-cardiac factors. Moreover, although ACP could be contributed by volume overload, we feel that this would be partially mitigated by our ICU’s fluid management protocol, which we had established since 2011 [13]. Also, although we cannot completely exclude a cardiac contribution to ACP, the overlap between left ventricular ejection fraction < 40% and ACP was only 10 patients, which was 15.2% of the 66 patients with ACP. We also did a sensitivity analysis for the presence or absence of left ventricular ejection fraction < 40%, using logistic regression with respect to ICU and hospital mortality, adjusted for age and APACHE II score. Including patients with left ventricular ejection fraction < 40%, ACP was associated with ICU and hospital mortality with an adjusted odds ratio of 2.14 (95% CI 1.13–4.04) and 1.89 (1.02–3.50), respectively. Excluding patients with left ventricular ejection fraction < 40%, ACP was associated with ICU and hospital mortality with an adjusted odds ratio of 2.38 (95% CI 1.17–4.84) and 1.88 (0.95–3.71), respectively. Therefore, the presence of left ventricular ejection fraction < 40% did not substantially alter the conclusions of our study.
Knowledge of the presence of ACP may be key to improving the survival of ARDS patients [5, 24]. To this end, Mekontso-Dessap and colleagues found that four variables could be used to risk-stratify ARDS patients for the presence of ACP (as determined by transesophageal echocardiography within three days of ARDS diagnosis): pneumonia as a cause of ARDS, driving pressure ≥ 18 cm H2O, PF ratio < 150 mmHg and arterial carbon dioxide partial pressure ≥ 48 mmHg [3]. Among our patients (Table 1), arterial carbon dioxide partial pressure was indeed significantly higher in patients with severe ACP, though we did not detect significant differences in PF ratio, pneumonia diagnosis or driving pressure. Nonetheless, to use this four-variable risk stratification method, arterial blood gases must be drawn and that patients had to be well sedated or even paralyzed for accurate measurement of driving pressure. Furthermore, after risk stratification, confirmation by echocardiography would still be required. Based on our study, we suggest an alternative approach of directly screening all ARDS patients with BCCE, which we believe can be done quickly at the bedside.
In addition, we found that the presence of severe ACP can significantly add to the Berlin Definition for ARDS, and should not be taken as a mere marker of ARDS severity. Previously, the ARDS Definition Task Force reported that using the Berlin Definition, mild, moderate and severe ARDS were associated with hospital or 90-day mortality of 27% (95% CI 24–30%), 32% (95% CI 29–34%) and 45% (95% CI 42–48%), respectively [17]. In our cohort of patients with ARDS, the presence of severe ACP appears to upstage ARDS severity by one level—this may have implications on treatment thresholds and patient recruitment for future studies.
Our results suggest that screening of patients on admission, rather than waiting for clinical deterioration, would be preferable for early identification and treatment of abnormalities. For instance, the detection of severe ACP in ARDS patients should prompt strategies to protect the right ventricle. Such strategies include targeting plateau pressures below 27 cm H2O, maintaining adequate oxygenation and avoiding hypercarbia beyond 60 mmHg [2, 5]. Prone positioning to off-load the right ventricle and extracorporeal carbon dioxide removal to allow tidal volume (and hence plateau pressure) reduction could also be considered [5, 6]. However, while we encourage BCCE, it should only be done if frontline physicians are competent in its use and interpretation [25]. Moreover, it is a complementary modality and does not replace good clinical acumen and practice.
We acknowledge limitations for our study. Firstly, we performed a single-centre observational study, and our results require external validation. Secondly, due to resource limitations, we only managed to screen patients once within 48 h of admission and do not know whether a narrower screening interval (e.g. within 24 h of admission) or repeated screening after that would yield further information. Thirdly, we did not utilize transesophageal echocardiography as our ICU physicians have not acquired this level of expertise, and transesophageal echocardiography may improve the detection of severe ACP compared to using transthoracic echocardiography. Fourthly, although we checked that no patient had any pre-existing cor pulmonale clinically or on prior echocardiography (which was available for 63.6% of ACP cases), some patients might have developed subclinical cor pulmonale after their last echocardiography. Fifthly, we concede that determination of both LVEF and ACP may be imperfect. Nonetheless, in our experience, accuracy of visual LVEF grading and visual estimation of RV/LV size ratio were fairly good, even for trainees when compared with an experienced supervisor (correct grading achieved in 85% of cases for visual LVEF and in 92.5% of cases for visual estimation of RV/LV size ratio, after performing 30 echo examinations) [9]. Finally, we did not mask BCCE findings from clinicians, which meant that BCCE could have changed management. We did not study specific treatments administered, but should they improve survival, the association of BCCE-detected abnormalities with mortality would then be biased towards the null.
In conclusion, severe ACP—but not left ventricular dysfunction—may help identify ARDS patients at elevated risk of ICU and hospital mortality. BCCE, when used as a screening tool, can then alert the treating physician to the presence of ACP, allowing prompt institution of measures that may alter ARDS outcomes. While further validation is required, we believe that our study should encourage ICU physicians to incorporate BCCE into routine screening of ARDS patients admitted to ICU.
Authors’ contributions
KCS, JN, WTS, VO and JP jointly conceived the study and prepared the manuscript; KCS, JN, WTS and VO performed the data extraction; KCS performed the data analysis; JP supervised the analysis and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. Chan Yiong Huak, Yong Loo Lin School of Medicine, National University of Singapore, for providing independent statistical review of this manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
No consent to share data could be obtained.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Our Ethics Review Board (National Healthcare Group Domain-Specific Review Board) approved the study (Approval Number DSRB B/2013/00132). As the study is an observational one, the need for patient consent was waived.
Funding
No funding was required for this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACP
acute cor pulmonale
- APACHE
acute physiology and chronic health evaluation
- BCCE
basic critical care echocardiography
- ICU
intensive care unit
- PF ratio
ratio of arterial oxygen partial pressure to inspired oxygen fraction
Contributor Information
Kay Choong See, Email: kay_choong_see@nuhs.edu.sg.
Jeffrey Ng, Email: jeffrey_sk_ng@nuhs.edu.sg.
Wen Ting Siow, Email: wtsiow@gmail.com.
Venetia Ong, Email: venetia_ong@nuhs.edu.sg.
Jason Phua, Email: jason_phua@nuhs.edu.sg.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Vieillard-Baron A, Price LC, Matthay MA. Acute cor pulmonale in ARDS. Intensive Care Med. 2013;39(10):1836–1838. doi: 10.1007/s00134-013-3045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mekontso Dessap A, Boissier F, Charron C, Begot E, Repesse X, Legras A, Brun-Buisson C, Vignon P, Vieillard-Baron A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42(5):862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 4.Boissier F, Katsahian S, Razazi K, Thille AW, Roche-Campo F, Leon R, Vivier E, Brochard L, Vieillard-Baron A, Brun-Buisson C, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 5.Repesse X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest. 2015;147(1):259–265. doi: 10.1378/chest.14-0877. [DOI] [PubMed] [Google Scholar]
- 6.Guerin C, Matthay MA. Acute cor pulmonale and the acute respiratory distress syndrome. Intensive Care Med. 2014;42(5):934–936. doi: 10.1007/s00134-015-4197-z. [DOI] [PubMed] [Google Scholar]
- 7.Kanji HD, McCallum J, Sirounis D, MacRedmond R, Moss R, Boyd JH. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care. 2014;29(5):700–705. doi: 10.1016/j.jcrc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE, Tayal VS, Sullivan DM, Kline JA. Randomized, controlled trial of immediate versus delayed goal-directed ultrasound to identify the cause of nontraumatic hypotension in emergency department patients. Crit Care Med. 2004;32(8):1703–1708. doi: 10.1097/01.CCM.0000133017.34137.82. [DOI] [PubMed] [Google Scholar]
- 9.See KC, Ong V, Ng J, Tan RA, Phua J. Basic critical care echocardiography by pulmonary fellows: learning trajectory and prognostic impact using a minimally resourced training model. Crit Care Med. 2014;42(10):2169–2177. doi: 10.1097/CCM.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 10.Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, Tsung JW, Neskovic AN, Price S, Oren-Grinberg A, et al. International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr. 2014;27(7):683.e1–683.e33. doi: 10.1016/j.echo.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Vieillard-Baron A. Assessment of right ventricular function. Curr Opin Crit Care. 2009;15(3):254–260. doi: 10.1097/MCC.0b013e32832b70c9. [DOI] [PubMed] [Google Scholar]
- 12.Vieillard-Baron A, Prin S, Chergui K, Dubourg O, Jardin F. Echo–Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am J Respir Crit Care Med. 2002;166(10):1310–1319. doi: 10.1164/rccm.200202-146CC. [DOI] [PubMed] [Google Scholar]
- 13.See KC, Mukhopadhyay A, Lau SC, Tan SM, Lim TK, Phua J. Shock in the first 24 h of intensive care unit stay: observational study of protocol-based fluid management. Shock. 2015;43(5):456–462. doi: 10.1097/SHK.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med. 2012;40(3):725–730. doi: 10.1097/CCM.0b013e31823778ee. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 16.Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev. 2010;(7):CD002787. [DOI] [PubMed]
- 17.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 18.Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, Barreau-Baumstark K, Castanier M, Papazian L, Roch A. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2012;40(5):1539–1545. doi: 10.1097/CCM.0b013e3182451b4a. [DOI] [PubMed] [Google Scholar]
- 19.Brown SM, Pittman J, Miller Iii RR, Horton KD, Markewitz B, Hirshberg E, Jones J, Grissom CK. Right and left heart failure in severe H1N1 influenza A infection. Eur Respir J. 2011;37(1):112–118. doi: 10.1183/09031936.00008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boissier F, Razazi K, Seemann A, Bedet A, Thille AW, de Prost N, Lim P, Brun-Buisson C, Mekontso Dessap A. Left ventricular systolic dysfunction during septic shock: the role of loading conditions. Intensive Care Med. 2017;43(5):633–642. doi: 10.1007/s00134-017-4698-z. [DOI] [PubMed] [Google Scholar]
- 21.Sevilla Berrios RA, O’Horo JC, Velagapudi V, Pulido JN. Correlation of left ventricular systolic dysfunction determined by low ejection fraction and 30-day mortality in patients with severe sepsis and septic shock: a systematic review and meta-analysis. J Crit Care. 2014;29(4):495–499. doi: 10.1016/j.jcrc.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Huang SJ, Nalos M, McLean AS. Is early ventricular dysfunction or dilatation associated with lower mortality rate in adult severe sepsis and septic shock? A meta-analysis. Crit Care. 2013;17(3):R96. doi: 10.1186/cc12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36(6):1701–1706. doi: 10.1097/CCM.0b013e318174db05. [DOI] [PubMed] [Google Scholar]
- 24.Biswas A. Right heart failure in acute respiratory distress syndrome: an unappreciated albeit a potential target for intervention in the management of the disease. Indian J Crit Care Med. 2015;19(10):606–609. doi: 10.4103/0972-5229.167039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayo PH, Maury E. Echography is mandatory for the initial management of critically ill patients: we are not sure. Intensive Care Med. 2014;40(11):1760–1762. doi: 10.1007/s00134-014-3460-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No consent to share data could be obtained.


