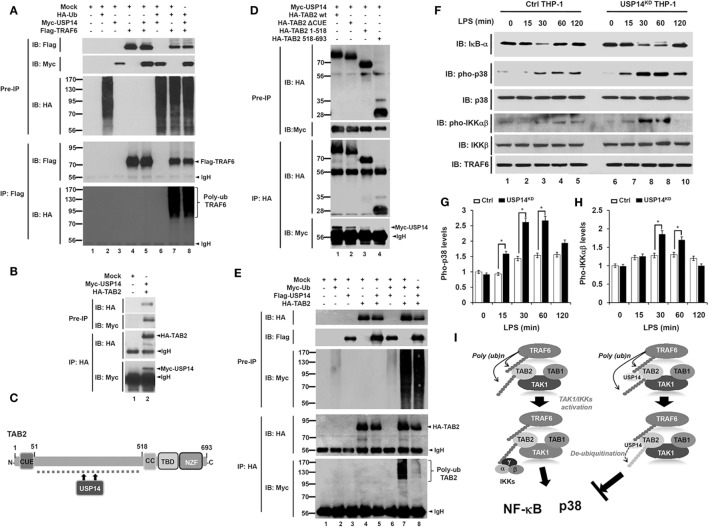
Figure 6.
Ubiquitin-specific protease 14 (USP14) induces deubiquitination of TAB 2 and inhibits toll-like receptor 4 (TLR4)-mediated signaling. (A) Myc-tagged USP14, HA-tagged Ub, Flag-tagged tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), and mock as control plasmid were co-transfected as indicated. At 38 h post-transfection, transfected cells were extracted, immunoprecipitated with anti-Flag antibody, and subjected to IB assay using anti-Flag, anti-Myc, or anti-HA antibody. (B) HEK293T cells were transiently transfected with mock as control plasmid, Myc-tagged USP14, or HA-tagged TAB 2. After 38 h, IP assay was performed using anti-HA antibody followed by IB assay using anti-Myc or anti-HA antibody. (C) A schematic model showing interactions between TAB 2 and USP14. (D) HA-tagged TAB 2 wild type (wt), HA-tagged TAB 2 truncated mutants, and mock as control plasmid were co-transfected with Myc-tagged USP14 into HEK293T cells followed by immunoprecipitation and western blotting analysis. (E) HEK293T cells were co-transfected with mock as control plasmid, Myc-tagged Ub, Flag-tagged USP14, or HA-tagged TAB 2 as indicated. At 38 h post-transfection, cells were extracted and immunoprecipitated with anti-HA antibody followed by an IB assay using anti-Myc, anti-Flag, or anti-HA antibody. (F) Ctrl THP-1 and USP14KD THP-1 cells were treated with or without LPS (200 ng/ml) for different time periods and then western blot assay was performed using anti-IκB-α, anti-pho-p38, anti-p38, anti-pho-IKKαβ, anti-IKKβ, and anti-TRAF6 antibody. (G,H) Band intensity of pho-p38 (G) and pho-IKKαβ (H) were analyzed with Image J (bottom). Data shown are averages from a minimum of three independent experiments. *P < 0.05. (I) A schematic model showing how USP14 regulates TLR4 signaling. Following TLR4 stimulation, ubiquitinated TRAF6 is associated with the TAB 2–TAK1–TAB 1 complex through interaction between its polyubiquitinated chain and TAB 2. TAB 2 is then ubiquitinated and the complex then facilitates the activation of TAK1. Simultaneously, the IKK complex is associated with the former complex through polyubiquitinated chain and TAB 2, leading to the activation of nuclear factor-kappa B (NF-κB) and p38 (left). In contrast, interaction between TAB 2 and USP14 may lead to deubiquitination of TAB 2, which inhibits the association of IKKs, thereby inhibiting the activation of NF-κB and p38 (right).