Fig. 10.
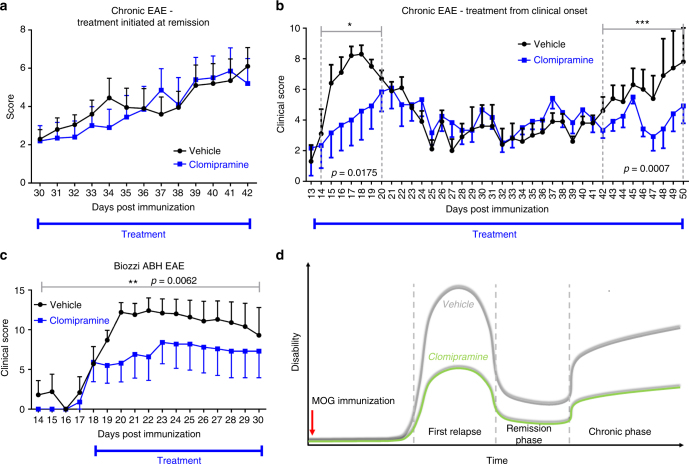
Clomipramine improves the chronic phase of EAE. a Female C57BL/6 (age 8–10 weeks) MOG-immunized mice were treated with clomipramine IP (25 mg/kg) or PBS (vehicle) from remission after the first relapse, and this did not affect disease score between the groups (n = 10 vehicle, n = 10 clomipramine). b In a second experiment, MOG-immunized C57BL/6 mice were treated from onset of clinical signs. Here, clomipramine reduced the clinical severity of the first relapse (day 14–20, p = 0.0175, two-tailed Mann–Whitney t-test) and of the second relapse at the late chronic phase (day 42–50, p = 0.0007, two-tailed Mann–Whitney t-test) (n = 5 vehicle, n = 6 clomipramine). Note that an initial two-way ANOVA with Sidak's multiple-comparisons test of the experiment from day 13–50 was not statistically significant, since vehicle-treated mice spontaneously remitted to a very low disease score between days 25 and 42, so that differences with the treatment group could not be detected. Hence, we analyzed differences of the acute and chronic relapse phases outside of the period of remission, using Mann–Whitney t-test. c Using Biozzi ABH mice, treatment from onset of clinical disability showed a positive effect on the chronic phase (p = 0.0062, two-tailed Mann–Whitney test) (n = 5 vehicle, n = 5 clomipramine). When a two-way ANOVA with Sidak's multiple-comparisons test was used, the results were not significant since the individual variability of mice in either group in any given day was very high for this model in our hands. d A summary of the effect of clomipramine when treatment is initiated at the onset of clinical signs