Fig. 8.
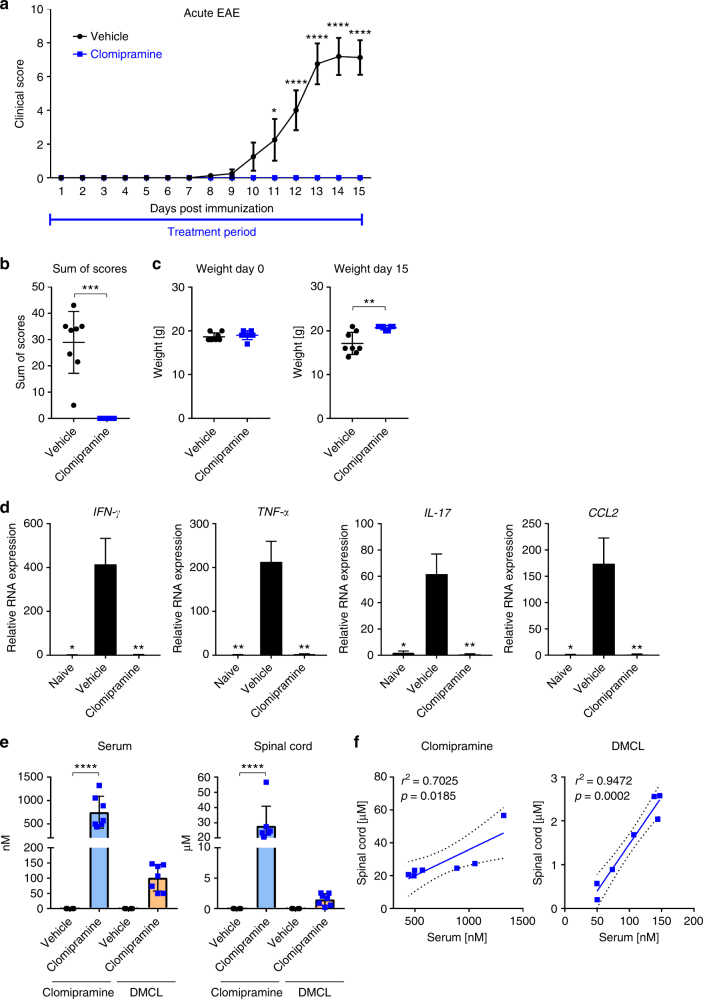
Early clomipramine treatment suppressed EAE disease activity. Female C57BL/6 mice (age 8–10 weeks) were treated with clomipramine IP (25 mg/kg) or PBS (vehicle) from the day of induction of MOG-EAE (day 0). From day 11 the clinical course differed significantly (p < 0.05); while vehicle-treated mice accumulated progressive disability, clomipramine-treated mice remained unaffected even up to the termination of the experiment when vehicle-treated mice were at peak clinical severity (paralysis or paresis of tail and hind limb functions and paresis of forelimbs) (a). The overall burden of disease per mouse was plotted in b, while the relative weight of mice, reflecting general health, is shown in c. In the lumbar cord at animal sacrifice (day 15), there was a significant upregulation in vehicle-EAE mice of transcripts encoding Ifng, Tnfa, Il-17, and Ccl2 compared to naïve mice, whereas clomipramine-treated mice did not show these elevations (d). Levels of clomipramine and the active metabolite desmethylclomipramine in serum and spinal cord at sacrifice (e) are consistent to concentrations reached in humans. There was a strong correlation of serum levels of clomipramine and desmethylclomipramine with spinal cord levels (f). Data in d are RT-PCR results, with values normalized to Gapdh as housekeeping gene and expressed in relation to levels in naïve mice. n = 8 vehicle and n = 7 clomipramine EAE mice. Data are depicted as mean ± SEM. Two-way ANOVA with Sidak's multiple-comparisons test as post hoc analysis (a), two-tailed unpaired non-parametric Mann–Whitney test (b), two-tailed unpaired t-test (c, e, f), and one-way ANOVA with Tukey's multiple comparisons test as post hoc analysis (d). Correlations were calculated using a linear regression model, dotted lines show the 95% confidence interval (f). Significance is shown as *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001