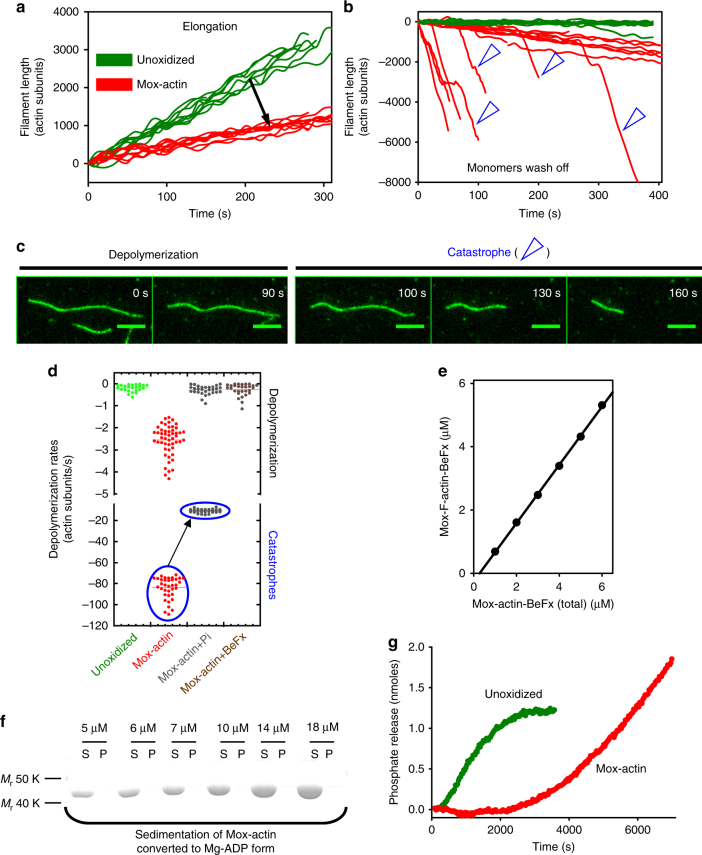
Fig. 1.
Mical-oxidation of actin induces its nucleotide state-dependent catastrophic disassembly. a Mical oxidation of actin inhibits filament elongation. Average elongations rates of unoxidized and Mical-oxidized (Mox) actin filaments (1.24 µM, 15% labeled with Alexa488-SE) were 11.4 ± 0.7 subunits/s (mean ± standard deviation (s.d.); n = 10 filaments) and 3.6 ± 0.4 subunits/s (s.d.; n = 20 filaments), respectively. b, c Mox-F-actin undergoes nucleotide state-dependent catastrophic disassembly in the absence of monomers. b Mox-actin filaments (red) depolymerize faster than unoxidized F-actin (green) in single filament TIRF assays. Catastrophes are indicated with open blue arrowheads. c TIRFM image montages of a single Mox-actin filament switching from depolymerization to the catastrophic disassembly phase. d Inorganic phosphate (Pi) reduces the rates of Mox-actin depolymerization and BeFx (mimics ADP-Pi state) abolishes catastrophic disassembly events (encircled in blue). Single filament depolymerization rates were determined from TIRF movies (see Table 1). e Mical-oxidized F-actin complex formation with BeFx reduces the critical concentration (Cc) of Mox-actin (0.24 ± 0.05 μM; mean ± s.d.; n = 3 independent measurements) compared to that of its mixed nucleotide state (~ 1 μM)13. f Mical-oxidized ADP-F-actin is highly unstable. Mox-F-actin disassembles when converted to the ADP form. Based on high-speed sedimentation experiments, no ADP-Mox-F-actin was detected in pellets (up to 18 μM concentration); S soluble (G-actin), P pellet (F-actin). n = 3 independent experiments. The unprocessed original scan of the gel is shown in Supplementary Fig. 8. g Mox-F-actin (red) shows non-saturating kinetics of phosphate release, which is indicative of rapid actin turnover (disassembly and reassembly). Phosphate release from unoxidized actin plateaus as expected (green). Average of three kinetic measurements is shown for each condition