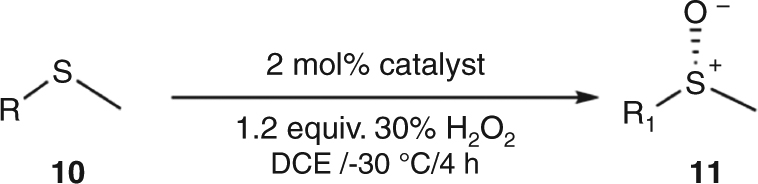
Table 4.
Oxidation of sulfide catalyzed by 1-Mn a
| |||||
|---|---|---|---|---|---|
| Entry | R | Catalyst | Conv. (%)b | Yield (%)c | ee (%)d |
| 1 | Ph | 1-Mn | 99 | 93 | 92 (S) |
| 2e | Ph | Homo | 71 | 67 | 0 |
| 3 | 4-MePh | 1-Mn | 99 | 93 | 92 (S) |
| 4e | 4-MePh | Homo | 63 | 59 | 0 |
| 5 | 4-MeOPh | 1-Mn | 95 | 92 | 84 (S) |
| 6e | 4-MeOPh | Homo | 80 | 75 | 0 |
| 7 | 4-ClPh | 1-Mn | 96 | 94 | 89 (S) |
| 8 | 3-FPh | 1-Mn | 98 | 91 | 87 (S) |
a Reaction conditions: 10 (0.1 mmol), (S)-1-Mn (2 mol % catalyst based on MOF), 1.2 equiv. aqueous H2O2 in DCE (0.8 mL), −30 °C, 4 h. No over-oxidized sulfone byproducts were detected
b Determined by 1H NMR analysis
c Isolated yield
d Determined by chiral HPLC analysis (letters in brackets specify the excess isomer)
e A 1:1 mixture of MnCl2 ·4H2O and (S)-Me2 L (6 mol% catalyst based on Mn) was used as the catalyst
