FIG 4.
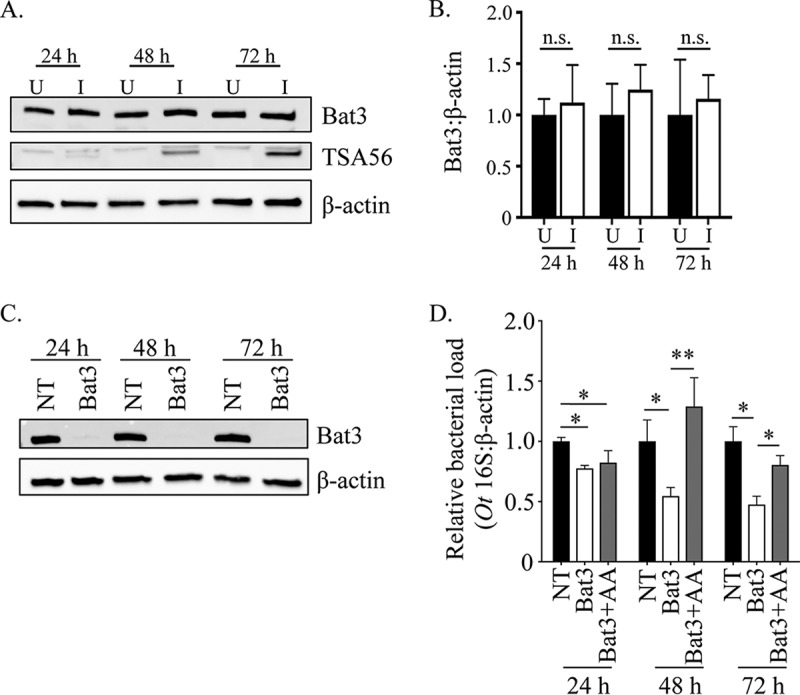
The timing of O. tsutsugamushi-mediated ERAD inhibition is important for bacterial growth. (A and B) O. tsutsugamushi infection does not alter Bat3 cellular levels. (A) Whole-cell lysates of O. tsutsugamushi infected (I) or uninfected (U) HeLa cells were subjected to Western blotting using Bat3, TSA56, and β-actin antibodies. (B) Densitometry was performed on blots from three separate experiments. (C and D) Inhibition of O. tsutsugamushi growth resulting from siRNA-mediated knockdown of Bat3 can be rescued by free amino acid supplementation. HeLa cells were treated with Bat3-targeting (Bat3) or nontargeting (NT) siRNA for 48 h, followed by infection with O. tsutsugamushi. Bat3-targeting siRNA-treated cells were cultivated in media that were either supplemented with amino acids (+AA) or not, while nontargeting siRNA-treated infected cultures were grown in medium alone. At 24, 48, and 72 h postinfection, total DNA was isolated or whole-cell lysate was generated from each sample. (C) Western blotting of whole-cell lysates of treated and control cells were screened with antibody against Bat3 or host β-actin to confirm knockdown. (D) Relative levels of the O. tsutsugamushi 16S rRNA gene were normalized to levels of β-actin by using the 2−ΔΔCT method. Resulting relative levels per treatment were normalized to those of the respective vehicle control at each time point. Statistically significant values are indicated: *, P < 0.05; **, P < 0.01; n.s., not significant. Results are representative of at least three separate experiments performed in triplicate.
