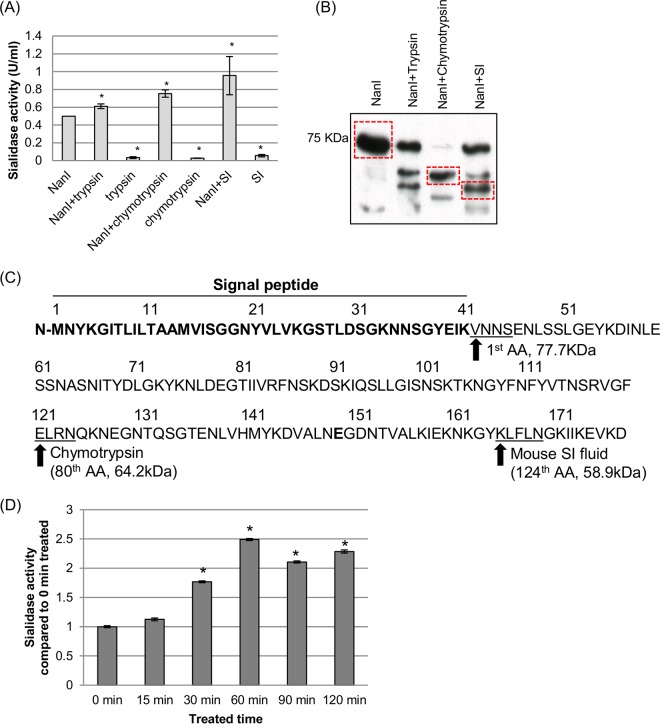
FIG 4.
Proteolytic processing of NanI sialidase. (A and B) Purified NanI (0.5 U/ml) was digested with chymotrypsin (40 ng/ml), trypsin (40 ng/ml), or mouse small intestinal fluid (0.4 μl/μl) at 37°C for 1 h. Following these digestions, samples were assayed for sialidase activity (A) and subjected to SDS-PAGE and NanI Western blotting (B). (C) The dominant digested NanI species present in the chymotrypsin and mouse SI fluid digestions, as noted by boxes in panel B, were selected for N-terminal sequencing to determine the N-terminal cleavage site for each species. AA, amino acid. (D) Kinetics of NanI activity in the presence of mouse SI fluid. The averages of data from three replications are shown in panels A and D, and a representative Western blot is depicted in panel B. In panels A and D, * indicates a significant (P < 0.05) difference compared to wild-type NanI. Error bars show standard deviations.