Table 1b.
Overview of the population pharmacokinetic models used for the evaluation of drug–drug interactions of phenobarbital, phenytoin, topiramate, valproate and zonisamide
| Model | Phenobarbital | Phenytoin | Topiramate | Valproate Adults | Valproate Children | Zonisamide |
|---|---|---|---|---|---|---|
| First author | Goto 20 | Odani 21 | Girgis 22 | Blanco‐Serrano 23 | Blanco‐Serrano 24 | Okada 25 |
| Population | Japanese | Japanese | NA (Caucasian presumably) | Spanish | Spanish | Japanese |
| Sample size (No. of patients) | 79 | 116 | 1217 | 255 | 208 | 99 |
| Sample size (No. of patients) | 260 | 531 | 4640 | 770 | 534 | 282 |
| Age (y) | 0.8–44 | 1–37 | 2–85 | 14–95 | 0.1–14 | 1.36–39.24 |
| Weight (kg) | 8–80 | 42.4 ± 16.5 | NA | 4–74 | 27–100 | 10–117 |
| Samples at | TDM | Peak/Trough | NA | TDM | TDM | 4.3 ± 2.8 h after dose |
| Graphical representation |
|
|
|
|
|
|
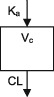
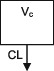
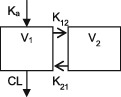
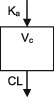
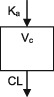
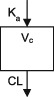
| Parameters | Ka, Vc, CL | Vc, CL (Vmax, Km) | Ka, Vss (V1 + V2), K12, K21, CL | Ka, Vc, CL | Ka, Vc, CL | Ka, Vc, CL |
| Between‐subject variability | Vc, CL | Vc, Vmax, Km | Ka, Vc, CL | CL | CL | CL |
| Covariate effects on CL | WT, PHT, VPA | WT, daily PHT dose, ZNS | Age, WT, Inducers (CBZ/PHB/PHT), VPA, NEMD, ZNS | WT, Dose, CBZ, PHT, PHB | WT, Dose, CBZ | WT, Dose, CYP2C19 genotype, CBZ, PHB, PHT |
| Covariate effects on V | ‐ | WT | WT | WT | WT | WT |
CL, clearance; Ka, absorption rate constant; Km, Michaelis‐Menten constant; K12/K21, distribution rate constants from and to central and peripheral compartments, respectively; NA, not available; TDM, therapeutic drug monitoring; V, volume of distribution; V1 / Vc, volume of distribution of the central compartment; V2, volume of distribution of the peripheral compartment, Vmax, maximum metabolic capacity; WT, weight
