Abstract
Background:
Osteoporosis is a common condition among the elderly population, and is associated with an increased risk of fracture. One of the most common fragility fractures involve the distal radius, and are associated with risk of subsequent fragility fracture. Early treatment with bisphosphonates has been suggested to decrease the population hip fracture burden. However, there have been no prior economic evaluations of the routine treatment of distal radius fracture patients with bisphosphonates, or the implications on hip fracture rate reduction.
Methods:
Age specific distal radius fracture incidence, age specific hip fracture rates after distal radius fracture with and without risendronate treatment, cost of risendronate treatment, risk of atypical femur fracture with bisphosphonate treatment, and cost of hip fracture treatment were obtained from the literature. A unique stochastic Markov chain decision tree model was constructed from derived estimates. The results were evaluated with comparative statistics, and a one-way threshold analysis performed to identify the break-even cost of bisphosphonate treatment.
Results:
Routine treatment of the current population of all women over the age of 65 suffering a distal radius fracture with bisphosphonates would avoid 94,888 lifetime hip fractures at the cost of 19,464 atypical femur fractures and $19,502,834,240, or on average $2,186,617,527 annually, which translates to costs of $205,534 per hip fracture avoided. The breakeven price point of annual bisphosphonate therapy after distal radius fracture for prevention of hip fractures would be approximately $70 for therapy annually.
Conclusion:
Routine treatment of all women over 65 suffering distal radius fracture with bisphosphonates would result in a significant reduction in the overall hip fracture burden, however at a substantial cost of over a $2 billion dollars annually. To optimize efficiency of treatment either patients may be selectively treated, or the cost of annual bisphosphonate treatment should be reduced to cost-effective margins.
Keywords: Bisphosphonates, Distal radius fracture, Hip fracture, Osteoporosis, Risendronate
Introduction
Osteoporosis is a common condition among the elderly, and has an increasing prevalence due to the growing elderly population. Over 50% of men and women over the age of 80 meet diagnostic criteria for osteoporosis (1). The disease burden of osteoporosis manifests itself as fragility fractures, which poses both significant social and economic implications (2-4). One of the most common fragility fractures involve the distal radius, which is often the index fracture among patients suffering from osteoporosis (5, 6). Prior fracture of the distal radius is associated with significant increased risk of subsequent fragility fracture (3, 7-11).
Bisphosphonates have emerged as a treatment for fragility fracture prevention in osteoporotic patients. Bisphosphonate therapy has demonstrated promise of significantly reducing the rate fractures in high risk patients, and in the appropriate patients can play an important role in long-term management of osteoporosis (12-16). Early initiation of treatment with bisphosphonates after fragility fracture has been suggested as a means of population hip fracture burden reduction (17-19). Some studies have suggested that broad utilization of bisphosphonates in the elderly and routine use after fragility fractures is cost effective (12, 20). In particular, distal radius fractures have been targeted as a point of intervention for osteoporosis treatment (8, 21). However, there have been no prior economic evaluations of the routine treatment of distal radius fracture patients with bisphosphonates, and the implications on hip fracture rate reduction.
Materials and Methods
Overview
We evaluated the economic implications of routine prescription of risendronate after distal radius fracture. We utilized a Markov chain cohort decision model based on outcomes and state transition probabilities obtained from the literature. Markov modeling allows the ability to follow a cohort or single patient through different states of health over fixed intervals of time, where the patient may either stay in a given health state or transition to another one, accruing outcomes at each interval (22, 23). The decision analysis was conducted with Monte Carlo simulation with cycle length of 1 year of a cohort representative of the US population distribution using TreeAge Pro (TreeAge Pro 2013, TreeAge Software, Williamstown, MA). Monte Carlo simulations, or stochastic simulations, rely on repeat sampling of probabilities to obtain distributions of outcomes; using this method with a Markov chain decision model allows simulation of individual patients across the model, incorporating variability at each point of probability and providing representative cohort wide outcomes (24).
Data
Literature estimates of age specific distal radius fracture incidence and age specific hip fracture rates after distal radius fracture with and without risendronate treatment were obtained (9, 15, 25, 26). The risk of atypical femur fracture with bisphosphonate treatment were also obtained (27).
The direct costs of hip fracture management were from the average reimbursements for DRG 482 and CPT 27245 obtained from public Medicare databases, and anesthesia reimbursements for CPT 01230 for a one hour case from the Medicare pricer. The cost of risendronate treatment was obtained from the literature.
Model
A unique stochastic Markov chain decision tree model was constructed from derived estimates. The tree was analyzed with a modified Monte Carlo simulation of a cohort of women 65 and older based of 2012 US population estimates (28). The results were evaluated with comparative statistics, and a one-way threshold analysis performed to identify the break-even cost of bisphosphonate treatment.
Results
Our model predicts 357,656 lifetime hip fractures after incidence of distal radius fracture in the cohort of all females aged over 65 in the US. In the same cohort of patients routinely treated with bisphosphonates after distal radius fracture, the number of hip fractures was predicted at 262,767 over the lifetime of the cohort, and was also associated with 19,464 atypical femur fractures [Figure 1].
Figure 1.
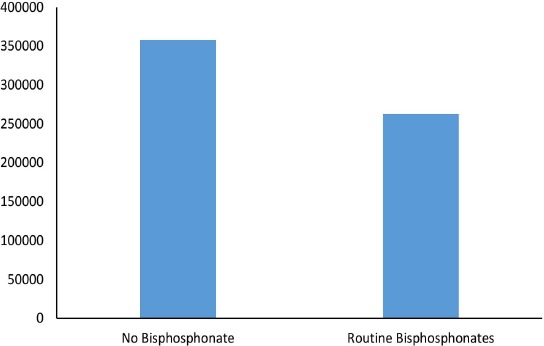
Lifetime Hip Fractures of the Cohort of Women >65 in the US.
The direct costs of hip fractures associated with routine non-treatment of osteoporosis after distal radius fracture was $4,165,256,788 over the lifetime of the cohort, or $466,131,380 on average annually. The direct costs of hip fractures, atypical fractures, and routine use of bisphosphonates over the lifetime of the same cohort was $23,668,091,028, or $2,652,748,908 on average annually [Figure 2]. The difference in cost is significant at α= 0.95.
Figure 2.
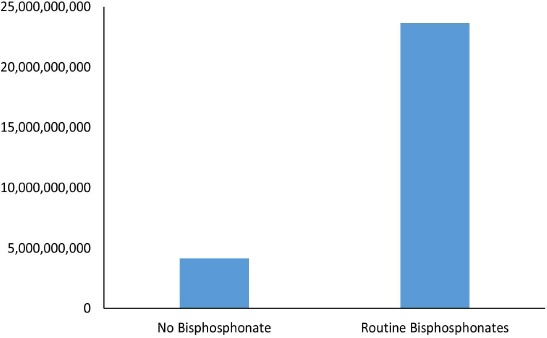
Lifetime Direct Costs of Hip Fractures and Osteoporosis Therapy of the Cohort of Women >65 in the US.
Sensitivity Analysis
One way sensitivity analysis was performed varying the cost of bisphosphonates to identify the threshold at which routine bisphosphonate use after distal radius fracture became cost neutral. We found that the breakeven price point of annual bisphosphonate therapy after distal radius fracture for prevention of hip fractures would be approximately $70 for therapy annually.
Discussion
Osteoporosis is a population health concern that particularly with the growing elderly population has tremendous clinical and economic implications (2, 3). Bisphosphonate therapy has been suggested as a means of helping manage the burden of fragility fractures in the elderly. Despite their beneficial impact on the prevention of fragility fractures, bisphosphonates are also associated with rare but concerning atypical femoral shaft fractures (27, 29, 30). While these fractures occur sporadically, they do offset the disease and economic benefits that may be accrued from avoiding fragility fractures. Furthermore bisphosphonate therapy in and of itself can be expensive. Utilization of bisphosphonates in post-menopausal women with DEXA positive osteoporosis is considered to be cost effective, and even more aggressive routine utilization of bisphosphonates after index fragility fracture has been suggested (8, 16, 21).
Here we present the first economic model evaluating wide-spread utilization of bisphosphonate therapy after distal radius fracture. Routine treatment of the current population of all women over the age of 65 suffering a distal radius fracture with bisphosphonates would avoid 94,888 lifetime hip fractures at the cost of 19,464 atypical femur fractures and $19,502,834,240, or on average $2,186,617,527 annually, which translates to costs of $205,534 per hip fracture avoided. So while significant risk reduction of hip fractures may be achieved with bisphosphonate use, this comes at a significant cost. We found the primary driver of the economic imbalance with routine bisphosphonate utilization in our model is the cost of the therapy itself, with some contribution from the incidence of atypical femur fractures. Driving the cost of therapy down towards the breakeven point of $70 annually would help avoid excess cost. Also selecting patients for therapy that may be at lower risk for atypical femur fracture, i.e. with anatomy consistent with larger neck-shaft angles, may reduce the consequent burden of “bisphosphonate fractures.” However given the significant excess cost per fracture prevented, we would not recommend routine prescription of bisphosphonate therapy after distal radius fracture, but instead follow-up with DEXA for evaluation of osteoporosis status prior to any pharmacological therapy.
References
- 1.Looker AC, Orwoll ES, Johnston CC, Jr, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12(11):1761–8. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–51. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(Suppl 6):S164–9. [PubMed] [Google Scholar]
- 4.Svedbom A, Ivergård M, Hernlund E, Rizzoli R, Kanis JA. Epidemiology and economic burden of osteoporosis in Switzerland. Arch Osteoporos. 2014;9(1):187. doi: 10.1007/s11657-014-0187-y. [DOI] [PubMed] [Google Scholar]
- 5.Mallmin H, Ljunghall S, Persson I, Bergström R. Risk factors for fractures of the distal forearm: a population-based case-control study. Osteoporos Int. 1994;4(6):298–304. doi: 10.1007/BF01622186. [DOI] [PubMed] [Google Scholar]
- 6.Bergström U, Björnstig U, Stenlund H, Jonsson H, Svensson O. Fracture mechanisms and fracture pattern in men and women aged 50 years and older: a study of a 12-year population-based injury register, Umeå, Sweden. Osteoporos Int. 2008;19(9):1267–73. doi: 10.1007/s00198-007-0549-z. [DOI] [PubMed] [Google Scholar]
- 7.Chen CW, Huang TL, Su LT, Kuo YC, Wu SC, Li CY, et al. Incidence of subsequent hip fractures is significantly increased within the first month after distal radius fracture in patients older than 60 years. J Trauma Acute Care Surg. 2013;74(1):317–21. doi: 10.1097/ta.0b013e31824bb325. [DOI] [PubMed] [Google Scholar]
- 8.Freedman BA, Potter BK, Nesti LJ, Cho T, Kuklo TR. Missed opportunities in patients with osteoporosis and distal radius fractures. Clin Orthop Relat Res. 2007;454(4):202–6. doi: 10.1097/01.blo.0000238866.15228.c4. [DOI] [PubMed] [Google Scholar]
- 9.Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. A meta-analysis. J Bone Joint Surg Am. 2003;85-A(10):1936–43. doi: 10.2106/00004623-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Hodsman AB, Leslie WD, Tsang JF, Gamble GD. 10-year probability of recurrent fractures following wrist and other osteoporotic fractures in a large clinical cohort: an analysis from the Manitoba Bone Density Program. Arch Intern Med. 2008;168(20):2261–7. doi: 10.1001/archinte.168.20.2261. [DOI] [PubMed] [Google Scholar]
- 11.Schousboe JT, Fink HA, Taylor BC, Stone KL, Hillier TA, Nevitt MC, et al. Association between self-reported prior wrist fractures and risk of subsequent hip and radiographic vertebral fractures in older women: a prospective study. J Bone Miner Res. 2005;20(1):100–6. doi: 10.1359/JBMR.041025. [DOI] [PubMed] [Google Scholar]
- 12.Tosteson AN, Burge RT, Marshall DA, Lindsay R. Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care. 2008;14(9):605–15. [PubMed] [Google Scholar]
- 13.Wells G, Cranney A, Peterson J, Joucher M, Shea B, Robinson V, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev. 2008;23(1):CD004523. doi: 10.1002/14651858.CD004523.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Sim IW, Ebeling PR. Treatment of osteoporosis in men with bisphosphonates: rationale and latest evidence. Ther Adv Musculoskelet Dis. 2013;5(5):259–67. doi: 10.1177/1759720X13500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins RB, Goeree R, Pullenayegum E, Adachi JD, Papaioannou A, Xie F, et al. The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord. 2011;12(1):209. doi: 10.1186/1471-2474-12-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleurence RL, Iglesias CP, Johnson JM. The cost effectiveness of bisphosphonates for the prevention and treatment of osteoporosis: a structured review of the literature. Pharmacoeconomics. 2007;25(11):913–33. doi: 10.2165/00019053-200725110-00003. [DOI] [PubMed] [Google Scholar]
- 17.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 18.Edwards BJ, Koval K, Bunta AD, Genuario K, Hahr A, Andruszyn L, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.”. J Bone Joint Surg Am. 2011;93(15):e87. doi: 10.2106/JBJS.I.00540. [DOI] [PubMed] [Google Scholar]
- 19.Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE. Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int. 2004;15(10):767–78. doi: 10.1007/s00198-004-1675-5. [DOI] [PubMed] [Google Scholar]
- 20.Thompson M, Pasquale M, Grima D, Moehrke W, Kruse HP. The impact of fewer hip fractures with risedronate versus alendronate in the first year of treatment: modeled German cost-effectiveness analysis. Value Health. 2010;13(1):46–54. doi: 10.1111/j.1524-4733.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 21.Freedman KB, Kaplan FS, Bilker WB, Strom BL, Lowe RA. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am. 2000;82-A(8):1063–70. doi: 10.2106/00004623-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Naimark D, Krahn MD, Naglie G, Redelmeier DA, Detsky AS. Primer on medical decision analysis: Part 5-Working with Markov processes. Med Decis Making.1997; 17(2):152–9. doi: 10.1177/0272989X9701700205. [DOI] [PubMed] [Google Scholar]
- 23.Detsky AS, Naglie G, Krahn MD, Redelmeier DA, Naimark D. Primer on medical decision analysis: Part 2-Building a tree. Med Decis Making. 1997;17(2):126–35. doi: 10.1177/0272989X9701700202. [DOI] [PubMed] [Google Scholar]
- 24.Krahn MD, Naglie G, Naimark D, Redelmeier DA, Detsky AS. Primer on medical decision analysis: part 4--analyzing the model and interpreting the results. Med Decis Making.1997; 17(2):142–51. doi: 10.1177/0272989X9701700204. [DOI] [PubMed] [Google Scholar]
- 25.Wilcke MKT, Hammarberg H, Adolphson PY. Epidemiology and changed surgical treatment methods for fractures of the distal radius: a registry analysis of 42,583 patients in Stockholm County, Sweden, 2004–2010. Acta Orthop. 2013;84(3):292–6. doi: 10.3109/17453674.2013.792035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams AL, Shi J, Takayanagi M, Dell RM, Funahashi TT, Jacobsen SJ. Ten-year hip fracture incidence rate trends in a large California population, 1997-2006. Osteoporos Int. 2013;24(1):373–6. doi: 10.1007/s00198-012-1938-5. [DOI] [PubMed] [Google Scholar]
- 27.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305(8):783–9. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 28.Annual Estimates of the resident population for selected age groups by sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: 2012 Population Estimates. United State Census Bureau. 2012 Available at: URL: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk .
- 29.Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28(8):1729–37. doi: 10.1002/jbmr.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pazianas M, Abrahamsen B, Wang Y, Russell RG. Incidence of fractures of the femur, including subtrochanteric, up to 8 years since initiation of oral bisphosphonate therapy: a register-based cohort study using the US MarketScan claims databases. Osteoporos Int. 2012;23(12):2873–84. doi: 10.1007/s00198-012-1952-7. [DOI] [PubMed] [Google Scholar]


