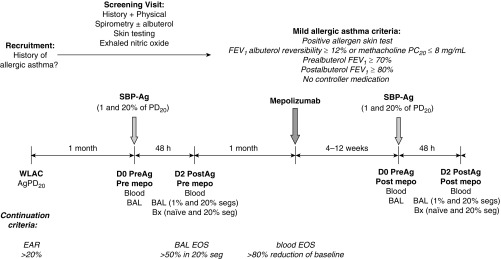
Figure 1.
Study design. This was an experimental, nonclinical study rather than a traditional pharmaceutical industry–sponsored “clinical trial.” Participants with mild allergic asthma underwent whole-lung allergen inhalation challenge (WLAC) to determine the allergen provocation dose resulting in a 20% reduction in FEV1 (AgPD20). An early allergic reaction (EAR), defined as a drop in FEV1 ≥ 20% within 1 hour of challenge, was required for continuation. One month later, bronchoscopy with bronchoalveolar lavage (BAL) was performed in two subsegments followed by segmental bronchoprovocation with allergen (SBP-Ag) at a dose of 1% (low dose) or 20% (high dose) of the subject’s AgPD20. Forty-eight hours later, bronchoscopy with BAL was performed in each subsegment and biopsies (Bx) were taken from an antigen-naive site and from the high-dose segment. BAL eosinophilia greater than 50% in the high-dose segment was required for continuation. One month after SBP-Ag, spirometry was performed to confirm continued asthma stability, and then a single 750-mg intravenous dose of mepolizumab was administered. One month after dosing, circulating eosinophils were required to be decreased by at least 80% of the baseline value, or fewer than 100 eosinophils/μl. If the threshold was not met, mepolizumab dosing was repeated. If the threshold was met, participants underwent bronchoscopy with BAL in two subsegments followed by SBP-Ag at the same 1 and 20% AgPD20 doses administered for the baseline challenge before mepolizumab. The postmepolizumab challenge was performed in the contralateral lung. Forty-eight hours after SBP-Ag, bronchoscopy with BAL was performed in each subsegment and Bx were taken from an antigen-naive site and from the high-dose segment. D0, D2 = Day 0, Day 2; EOS = eosinophils; mepo = mepolizumab; PC20 = provocative concentration of methacholine causing a 20% fall in FEV1; PD20 = provocative dose resulting in a 20% reduction in FEV1; PreAg = before antigen administration; PostAg = after antigen administration; seg/s = segment/s.