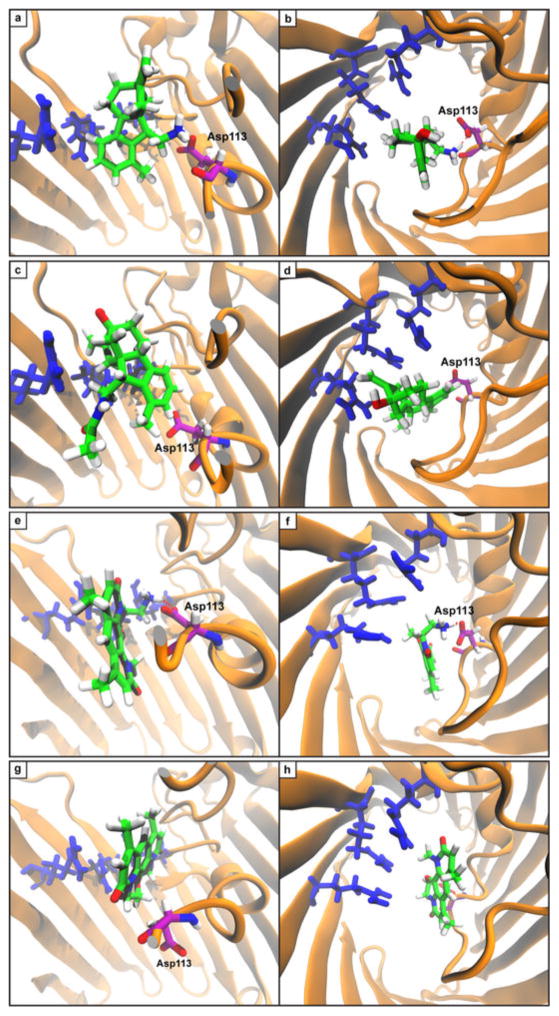
Extended Data Figure 7. Snapshots of SMD simulation of compound translocation through OmpF.
a, Compound 1 is capable of making a key interaction with Asp113 that assists in movement past constriction site. This finding is in accord with previous reports of the importance of Asp113 in producing the cation selectivity of OmpF63,64. b, Same pose as a, viewed from above. c, Compound 13 makes no interactions with Asp113 and thus faces additional barriers to penetrance. d, Same pose as c, viewed from above. e, 6DNM-NH3 makes key H-bonding interactions with Asp113 (purple) that assists in the molecule’s passage through the constriction site. f, Same pose as e, viewed from above. g, 6DNM requires larger movement by peptide backbone in order to pass through constriction site. Asp113 is appreciably displaced by 6DNM. h, Same pose as g, viewed from above. In all simulations, the largest barrier to translocation is a series of stacked arginine residues (Arg 42, 82, and 132) that are depicted in blue.