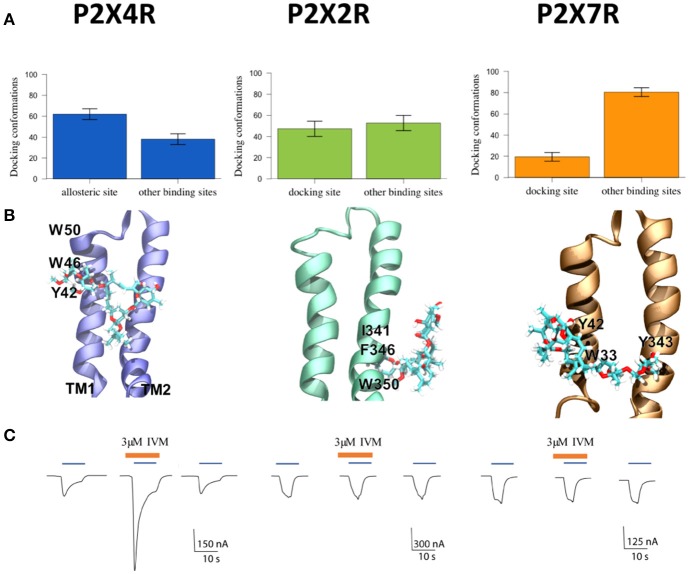
Figure 1.
Characterization of P2XR-IVM binding specificity by in silico and electrophysiological approaches. (A) Number of conformations obtained by molecular docking of IVM to rat P2X4R, P2X2R, and P2X7R. (B) Representative best energy conformation of IVM docking with the three P2XRs studied; lowest IVM P2X2R binding energy to other sites not analogous to the allosteric site in the P2X4R. IVM binding showed interaction with I341, F346, and W350 residues. In the case of P2X7R, IVM docking interacted with Y42, W33, and Y343. (C) Representative recordings of 1 μM ATP-gated currents in Xenopus laevis oocytes pretreated with 3 μM IVM (brown line) for 3 min prior to 1 μM ATP addition (blue line) in separate oocytes, each expressing P2X4R, or P2X2R, or P2X7R, respectively. Calibration scales are different for each oocyte-evoked currents during 10 s. *p < 0.05 between the allosteric or docking and other binding sites. #p < 0.05 between the allosteric site in the P2X4R and putative docking sites in the P2X2R or the P2X7R.