Key Points
Childhood HSCT survivors suffer from a higher burden of severe/life-threatening conditions compared to conventional therapy survivors.
Seven percent of HSCT survivors exhibit frailty phenotype at early age, placing them at higher risk for early mortality.
Abstract
Outcomes of hematopoietic stem cell transplantation (HSCT) have markedly improved over the past 2 decades, underscoring a need to better understand the long-term health effects of this intensive treatment modality. We describe the burden of chronic medical conditions and frail health among St. Jude Lifetime Cohort Study participants treated for childhood hematologic malignancies with HSCT (n = 112) or with conventional therapy (n = 1106). Chronic conditions and frail health were ascertained clinically and classified according to a modified version of the Common Terminology Criteria for Adverse Events (version 4.03) and the Fried Frailty Criteria. Seventy-nine transplants were allogeneic (41 from a sibling donor, 34 unrelated, and 4 others from related donor). Twenty-five allogeneic donor recipients had a history of chronic graft-versus-host disease. Compared to those treated with conventional therapy, a higher percentage of HSCT survivors had severe, disabling, or life threatening (grade 3-4) chronic conditions (81.3% vs 69.2%, P = .007). By age 25 years, HSCT survivors experienced 148 grade 3-4 events/100 compared to 60 among conventional therapy survivors (P < .001). Percentages of survivors with second neoplasms (17.0% vs 7.9%, P = .003), grade 3-4 cardiovascular (19.6% vs 10.2%, P = .004) and pulmonary (16.1% vs 4.6%, P < .001) conditions, and frail health (7.1% vs 1.6%, P < .001) were higher after HSCT than conventional therapy. These results underscore the need for clinical follow-up and provide data to guide the development of prevention and/or intervention strategies for this vulnerable population.
Visual Abstract
Introduction
A marked decrease in mortality following hematopoietic stem cell transplant (HSCT) during childhood has resulted in 1 year posttransplant survival rates of 80%.1,2 Although the probability of being alive 10 years after HSCT approximates 85% among children who survive 2 years,3 survivors are vulnerable to long-term morbidities. Here we describe the prevalence and burden of chronic medical conditions and frail health among adult St. Jude Lifetime Cohort Study4-6 participants who survived ≥10 years after treatment including HSCT (n = 112, 79 allogeneic, 33 autologous) between 1 January 1982 and 30 June 2005, for childhood hematological malignancies and compare them with those treated with conventional therapy (n = 1106).
Methods
Medical records were abstracted to obtain diagnosis and treatment data. The study protocol and documents are approved by the institutional review board and done in accordance with the Declaration of Helsinki. Laboratory and clinical examination data were used to classify 159 chronic conditions using a modified version of the Common Terminology Criteria for Adverse Events (version 4.03; supplemental Table 1).7,8 Cumulative burden of chronic disease was calculated using mean cumulative count; the mean number of recurrent/multiple health events experienced over time in the presence of competing events.9 The 5 Fried Frailty Criteria were used to classify prefrail (2 criteria) and frail (≥3 criteria) health.10
Results
In a clinically characterized cohort (supplemental Table 2; supplemental Figure 1) of survivors of childhood-onset hematological malignancies followed for an average of nearly 20 years, we found a similar prevalence of any grade 1-4 (100% among HSCT and 99.6% among conventional therapy recipients), but a higher prevalence of severe/disabling and life-threatening (grade 3-4) chronic health conditions (81.3 vs 69.2%, P = .007) when comparing HSCT recipients (mean age, 28.4 ± 5.9 years; mean time since diagnosis, 18.5 ± 4.2 years; 50.9% male) with those treated with conventional therapy (mean age, 29.3 ± 6.2 years; mean time since diagnosis, 19.9 ± 5.1 years; 52.5% male). HSCT recipients had more dyslipidemia (52.7 vs 37.3%, P = .002), lung disease (74.1 vs 26.8%, P < .001), cataracts (67.0 vs 14.3%, P < .001), osteonecrosis (20.5 vs 12.9%, P = .030), hepatic cirrhosis (6.3 vs 2.2%, P = .019), primary hypogonadism (30.4 vs 3.1%, P < .001), and secondary neoplasms (17.0 vs 7.9%, P = .003) than those treated with conventional therapy (supplemental Tables 2 and 3). Survivors treated with allogeneic HSCT had a higher prevalence of any (87.3% vs 66.7%, P = .011) and pulmonary (20.3% vs 6.1%, P < .001) grade 3-4 conditions than those treated with autologous HSCT. Accordingly, the prevalence of chronic conditions was higher among those whose conditioning included total body irradiation (supplemental Table 5).
Our data differ from previous investigations among HSCT survivors that have reported a lower prevalence of chronic health conditions.11-14 In the Bone Marrow Transplant Survivor Study (BMTSS), Armenian et al found a 79.0% prevalence of grade 1-4 and 25.5% of grade 3-4 self-reported chronic conditions among 145 childhood HSCT survivors (mean age, 24.0 years) surveyed 11 years post-HSCT.11 A Dutch study that used clinical data to ascertain chronic conditions among 162 survivors of childhood HSCT (median age, 13.5 years) with 7 years of follow-up reported a lower prevalence of any grade or grade 3-4 chronic conditions (93.2% and 25%, respectively).14 Our results likely differ from the BMTSS because we used clinical assessment rather than self-report to ascertain outcomes, and from both studies because of longer follow-up for the St. Jude Lifetime Cohort Study cohort.
Our data (supplemental Tables 3 and 4) extend previous literature describing the high risk for cardiovascular conditions, pulmonary impairment, and second neoplasms (SN) among HSCT survivors.11,13,15-17 Rates of severe/disabling and life-threatening cardiovascular events (19.6%) in our cohort were higher than those of childhood HSCT survivors in the BMTSS, in which 4.8% reported grade 3-5 cardiac events 11 years after HSCT.11 A novel finding in our pediatric HSCT survivor cohort is the mixed pattern of pulmonary impairment (9 of 11 survivors in our cohort with a grade 3 or 4 obstructive pulmonary deficit also had a restrictive and/or diffusion deficit) that is similar to that seen in HSCT survivors transplanted during adulthood. In a study of 593 adult 5-year survivors of allogeneic HSCT treated from 1980 through 1997, Marras et al noted that 4%, 7%, and 17% of participants had at least a 1 standard deviation decline in forced expiratory volume in 1 second/forced vital capacity, total lung capacity, and diffusion, respectively.18 The percentage who developed SN in our study (19.6%) was higher than that reported by the BMTSS (6.9% developed an SN 11 years post-HSCT)11 and by the Pediatric Oncology Group of Ontario Network Information System (3.1% of allogeneic, 2.5% of autologous HSCT recipients developed SN after 15 years of follow-up).17 Our higher percentages for SN are likely the result of longer follow-up.
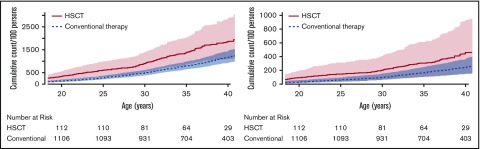
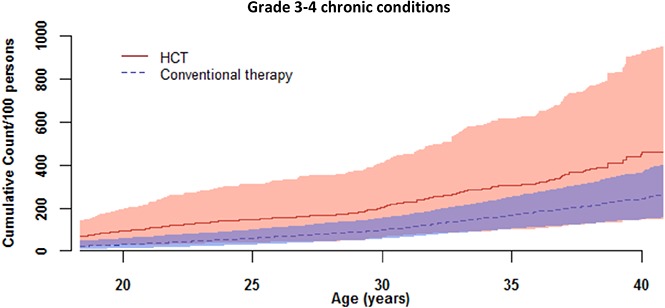
By age 25 years, the cumulative burden of chronic disease was 1.5 (ie, a survivor will, on average, experience 1.5 chronic diseases by age 25) for a survivor in our cohort treated with HSCT compared with 0.6 for a survivor treated with conventional therapy (Figure 1; supplemental Table 6). This overall disease burden was driven by high rates of hypertension, dyslipidemia, abnormal glucose metabolism, and obesity. These conditions contributed more than 20% of the total cumulative burden at age 25 years and indicate that traditional cardiovascular risk factors remain a significant issue in long-term childhood HSCT survivors. These data extend information provided by previous studies completed in childhood HSCT survivors earlier in follow-up after transplant, or in combined cohorts of pediatric and adult HSCT recipients. For example, Bajwa et al reported dyslipidemia in 13% of 160 childhood HSCT survivors 7 years after transplant19; Armenian et al, in a cohort of 1885 pediatric and adult HSCT survivors, reported 35.6% with hypertension, 16.8% with diabetes, and 43.7% with dyslipidemia 5 years posttransplant20; and Chow et al, in a cohort of 2362 pediatric and adult HSCT survivors, reported 33.9% with dyslipidemia, 27.9% with hypertension, and 14.3% with diabetes 11 years posttransplant.21
Figure 1.
Cumulative burden of chronic conditions among HSCT survivors compared with conventionally treated participants by attained age. (A) Grades 1-4 chronic burden curves. (B) Grades 3-4 chronic burden curves.
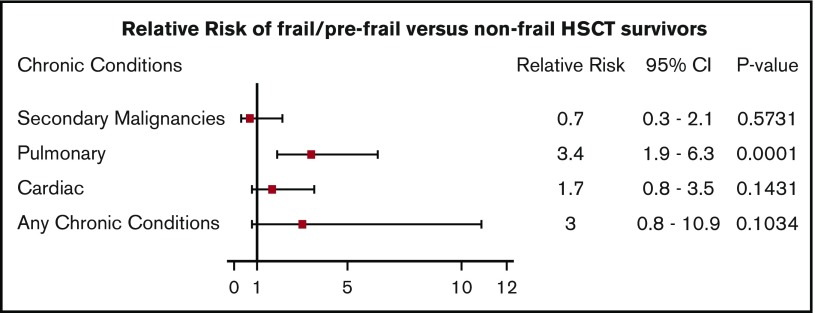
The prevalence of clinically ascertained frailty among adult survivors of childhood hematological malignancies treated with HSCT in this cohort was 7% compared with 1.6% among survivors treated with conventional therapy. This value is similar to the percentage (6.9%) observed among older adults (aged 65-101 years) in the general population,22 and was associated with a 3.5-fold increase in risk for severe, disabling, or life-threatening pulmonary disease (Figure 2). Initially described in geriatric populations, frailty is recognized as an important predictor of additional morbidity and mortality in childhood cancer survivors and in adult HSCT recipients.10,23 A recent study in adult HSCT survivors (mean age, 42 years; median time since transplant, 7.9 years) described a higher prevalence (8.0%) of frailty among HSCT survivors than among siblings (2.0%) and reported an association between frailty and mortality.23
Figure 2.
Relative risk of being prefrail/frail and having grade 3 or 4 cardiac, pulmonary, SN, or any chronic conditions.
Discussion
This study underscores that chronic disease burden and frail health are concerns for aging adult survivors of pediatric hematological malignancies treated with HSCT. Prospective clinical ascertainment of adverse outcomes is important. Interventions for modifiable conditions such as hypertension, dyslipidemia, abnormal glucose metabolism, and obesity may alter the course of more catastrophic sequelae such as cardiovascular and pulmonary disease. Early detection of second malignancies may improve long-term survival. Our data also indicate that survivors of childhood hematological malignancies treated with HSCT are at increased risk for frail health, which is of concern given the young age of our cohort. Further investigation in larger cohorts to determine the pathophysiology of, and to identify additional disease and treatment related risk factors for, this problem, and to determine potential intervention targets, is needed to optimize health in this vulnerable population.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Tracie Gatewood for her assistance preparing the letter.
Support for this study was provided by grants from the National Institutes of Health, National Cancer Institute (P30CA021765 and U01CA195547) (the latter to M.M.H. and L.L.R.) and American Lebanese Syrian Associated Charities.
Authorship
Contribution: K.K.N. conceived the study; H.M.E., L.L., and K.K.N. provided the study design and draft; L.L. and K.K.N. provided statistical analysis; H.M.E., L.L., M.B., N.B., M.J.E., B.M.T., and K.K.N. acquired data; M.M.H. and L.L.R. designed and conducted the St. Jude Lifetime Cohort Study cohort study; D.M.G. and D.A.M. participated in data analysis and interpretation; H.M.E., L.L., and K.K.N. interpreted data; and all authors contributed to article revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirsten K. Ness, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, MS-735, Memphis, TN 38105; e-mail: kiri.ness@stjude.org.
References
- 1.Pasquini MC, Zhu X. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Documents/2014_Summary_Slides.pptx. Accessed 15 January 2015.
- 2.Eapen M, Logan BR, Appelbaum FR, et al. Long-term survival after transplantation of unrelated donor peripheral blood or bone marrow hematopoietic cells for hematologic malignancy. Biol Blood Marrow Transplant. 2015;21(1):55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60(5):856-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mallah MH, Juraschek SP, Whelton S, et al. Sex differences in cardiorespiratory fitness and all-cause mortality: the Henry Ford ExercIse Testing (FIT) Project. Mayo Clin Proc. 2016;91(6):755-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. 2011;118(5):1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khera N, Storer B, Flowers ME, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30(1):71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129-3139, quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresters D, van Gils IC, Kollen WJ, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study. Bone Marrow Transplant. 2010;45(1):79-85. [DOI] [PubMed] [Google Scholar]
- 15.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant. 2013;19(7):1073-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socié G, Baker KS, Bhatia S. Subsequent malignant neoplasms after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S139-S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pole JD, Darmawikarta D, Gassas A, et al. Subsequent malignant neoplasms in pediatric cancer patients treated with and without hematopoietic SCT. Bone Marrow Transplant. 2015;50(5):721-726. [DOI] [PubMed] [Google Scholar]
- 18.Marras TK, Chan CK, Lipton JH, Messner HA, Szalai JP, Laupacis A. Long-term pulmonary function abnormalities and survival after allogeneic marrow transplantation. Bone Marrow Transplant. 2004;33(5):509-517. [DOI] [PubMed] [Google Scholar]
- 19.Bajwa R, Skeens M, Garee A, et al. Metabolic syndrome and endocrine dysfunctions after HSCT in children. Pediatr Transplant. 2012;16(8):872-878. [DOI] [PubMed] [Google Scholar]
- 20.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32(3):191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. [DOI] [PubMed] [Google Scholar]
- 23.Arora M, Sun CL, Ness KK, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2(10):1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.