Abstract
Although a significant increase in life expectancy for people with human immunodeficiency virus was reported last spring, experts in the U.S. caution that the results are not a cause for complacency. Efforts to develop a vaccine and a cure remain essential, as do efforts to develop interventions that may improve adherence.
In the spring of 2017, news outlets throughout the United States and Europe highlighted a study published in The Lancet HIV that reported a significant increase in life expectancy for people with human immunodeficiency virus (HIV)—particularly young adults just beginning antiretroviral treatment—between 1996 and 2013.1–3 Largely attributed to the availability of more effective and less toxic drugs, the study’s findings are encouraging in part because, as its authors note, they may help to reduce stigma associated with HIV infection and increase the likelihood that people with HIV will be able to obtain appropriate care, medical insurance, and employment.
However, while some of the study’s findings may be worthy of celebration, HIV experts in the U.S. caution that they are not a cause for complacency, especially in our current economic and political environment. Efforts to develop a vaccine and a cure remain essential, as do efforts to develop interventions that may improve adherence. Likewise, initiatives both inside and outside the U.S. that lead to earlier diagnoses and earlier treatment of those infected, while reducing the risk for new HIV infections, continue to be as critical as ever.4–6
The Current U.S. Landscape
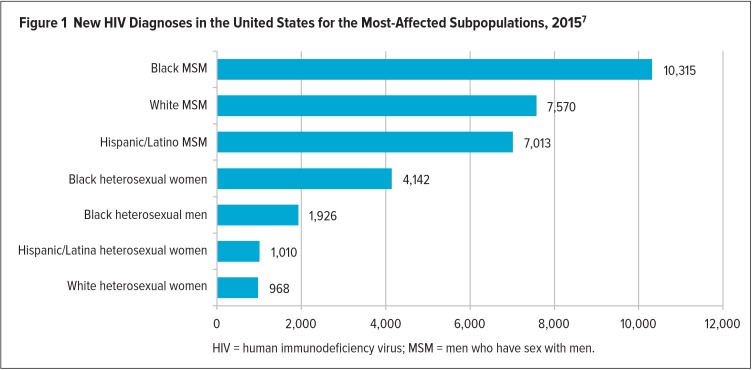
According to the most current data from the Centers for Disease Control and Prevention (CDC), approximately 1.1 million people in the U.S. are living with HIV, and a still-startling number of infected individuals—approximately one in seven—are unaware of their HIV status.7 In 2015, 39,513 people in the U.S. were diagnosed with HIV, representing a somewhat modest decline in estimated new infections per year of approximately 9% since 2010 (Figure 1).7 These statistics alone suggest that much work lies ahead on the road to achieving benchmarks established by the National HIV/AIDS Strategy (NHAS), which was released by President Obama in 2010 and updated in 2015.8 The 2015 update of the NHAS features a “90/90/90” goal for 2020—that is: 90% of people living with HIV will know their HIV status; 90% of individuals diagnosed with HIV will receive timely, high-quality, and uninterrupted HIV care; and 90% of people on antiretroviral therapy (ART) will achieve viral suppression (an undetectable level of virus in the blood) by 2020. Efforts to achieve these benchmarks, which are in line with global targets set forth in 2014 by the Joint United Nations Programme on HIV/AIDS (UNAIDS),9 may be further hampered by the January 2017 discontinuation of the Office of National AIDS Policy.
Figure 1.
New HIV Diagnoses in the United States for the Most-Affected Subpopulations, 20157
As clinicians, researchers, and others in the HIV/AIDS community strive to reach the 90/90/90 goal, aging with HIV has become a growing concern. Experts now anticipate that by 2020, more than 70% of Americans with HIV will be 50 years of age or older.10

Judith S. Currier, MD
“The treatment of HIV is certainly improving life span and allowing people with HIV to manage what is now essentially a chronic disease,” says Judith S. Currier, MD, Professor of Medicine, Chief of the Division of Infectious Diseases, and Co-Director of the Clinical AIDS Research and Education Center at the University of California in Los Angeles. “And, as people continue to live longer with the disease, patients and clinicians have become concerned about the long-term effects of living with HIV. We have a large population of people living with HIV now who do appear to be experiencing higher rates of several chronic diseases, such as cardiovascular disease, bone disease, and some types of cancer. A primary focus now is on trying to reduce morbidity related to these diseases.”
Long-term complications of HIV disease have been a growing focus of major international conferences. The 2017 Conference on Retroviruses and Opportunistic Infections (CROI), for instance, featured studies examining the impact and treatment of noncommunicable chronic diseases (e.g., cardiovascular disease, obesity, bone disease, and cancer) as well as opportunistic infections such as Mycobacterium tuberculosis and cryptococcosis.11 Related research has been examining the prevalence of and diagnostic, preventive, and treatment strategies for diabetes,12 opportunistic central nervous system infections, 13 kidney disease,14 stroke and other vascular disease,15 hepatitis B virus (HBV) and hepatitis C virus (HCV),16 and other diseases in HIV-infected populations.17
Dr. Currier is the incoming chair of the AIDS Clinical Trials Group (ACTG), one of the largest clinical trial networks in the world, which is sponsored by the National Institutes of Health (NIH). In her role as chair, Dr. Currier leads a steering committee that oversees a range of studies investigating aging and comorbidities in individuals with HIV, with a focus on identifying predictors of complications, as well strategies for prevention and treatment. One noteworthy NIH-sponsored trial (a collaboration between the ACTG and National Heart, Lung, and Blood Institute investigators) is the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE), which is expected to provide valuable information about inflammation and cardiovascular risk in HIV-infected individuals between 40 and 75 years of age.18
“This large clinical endpoint study will be a landmark trial to define whether guidelines for the use of statins should be different for people with HIV,” Dr. Currier says. “The study will examine whether it may be advantageous to begin statins earlier, and whether we may be able to reduce the risk of heart disease in HIV with statins. It is an important investment in our understanding of cardiovascular disease and other complications in people with HIV.”
One of the many goals of the REPRIEVE trial is to study gender differences in the onset, severity, and course of cardiovascular disease in individuals with HIV, as well as in individual responses to statin treatment. The trial will also examine how sex-specific factors, such as hormones, contribute to cardiovascular disease risk and/or risk reduction in individuals with HIV. The focus on women in this and other current HIV trials is particularly important: While approximately half of the 35 million to 40 million people in the world with HIV are women, women so far have been significantly underrepresented in HIV research.19
Progress in HIV Prevention
Another important area of HIV research—and one that also has researchers taking a closer look at gender differences—is that of prevention. While the search will continue for new protective mechanisms that may be used by individuals uninfected with HIV but at substantial risk of becoming infected, roughly a dozen clinical trials around the world have demonstrated that pre-exposure prophylaxis (PrEP)—antiretroviral retreatment in the form of a daily pill—is highly effective in limiting the transmission of HIV infection.20 In 2012, the Food and Drug Administration (FDA) approved tenofovir disoproxil fumarate (TDF) 300 mg/emtricitabine (FTC) 200 mg (Truvada, Gilead Sciences) for the prevention of HIV potentially acquired by any means, including sexual transmission or injectable drug use. Subsequently, guidelines for the provision of PrEP in clinical settings have been issued both inside and outside of the U.S., by major organizations such as the CDC and the World Health Organization. When taken correctly and with appropriate adherence, it has been estimated that PrEP may reduce an individual’s risk for acquiring HIV by as much as 92%.21
“In the absence of a vaccine, pre-exposure prophylaxis is an important way to give people the ability to take control of preventing HIV,” Dr. Currier says. “It clearly has been shown to be effective, and after a lot of work over the past few years we are starting to see, particularly in urban centers that have been on the forefront of rolling out PrEP, that it is truly having an impact, and that is an exciting development. Meanwhile, we are continuing to look into other novel types of pre-exposure prophylaxis, including agents that are antibodies, and other antiretroviral drugs and immune-based treatments that may be given less frequently.”
In the U.S., the delivery of HIV pre-exposure prophylaxis for women has faced a number of challenges, including a perception of limited risk of infection and a lack of awareness about PrEP on the part of both women and health care providers.22 Because about 19% of new infections are occurring in women, and because women account for about one-quarter of all individuals who have been diagnosed with HIV/AIDS,23 education focused on improving the awareness of risk as well as preventive strategies is essential. Studies of HIV prevention in women have also shown that women have a greater susceptibility to infection during unprotected sex compared with men, and women require dosing strategies and delivery systems that differ from those used by men.22,24 One important focus of current research is the delivery of PrEP during pregnancy.
“Our standard approach has been to test women during pregnancy,” Dr. Currier says, “and when women are found to be HIV positive, to start them on treatment right away to prevent transmission during birth. However, women who test negative may still be at risk of acquiring HIV, and this has been a significant concern because acute infection during pregnancy represents a high risk for transmitting virus to the baby. Because PrEP is a component of treatment, we have used these drugs in pregnant women before, but we hope to gather more information about the safety of their use in women who are at extremely high risk of seroconversion during pregnancy.”
PrEP and the continued investigation of novel preventive agents will remain critical until the availability of a vaccine and a cure, neither of which, despite huge investments,25,26 is expected to emerge within the next few years. As vaccine and cure research continue, both will require altruistic, highly motivated, well-informed volunteers who are willing to accept some degree of personal risk—although the scientific community is striving to reduce that risk through careful clinical trial design and monitoring.27
“It is important to clarify for individuals who may be contemplating participation in vaccine research that many vaccine trials examining efficacy are allowing participants to access PrEP,” Dr. Currier says.
“In our efforts to discover a cure,” she adds, “which involve testing interventions to reduce the size of the latent HIV reservoir, the only way we are going to know if they work is for people to interrupt their treatment. It’s usually possible to determine pretty quickly that an intervention hasn’t worked, since the virus will typically rebound in about four to 12 weeks. But there are safeguards in place, and interruption of treatment is done in a very controlled manner, so that for people who started treatment early and have been well suppressed, the short-term risks of being off treatment can be minimized.”
Aging Patients, Changing Care Needs
The aging of individuals with HIV, as well as changes in the prevention and treatment of HIV during the past decade, will likely require changes in the clinical oversight and management of the disease in the near future. Dr. Currier notes that it may be important for primary care providers—many of whom already have been assessing individuals at risk for the disease—to play a more integral role in disease management.
“And, with multiple diseases of aging,” she adds, “the involvement of specialists trained to manage those problems will become increasingly important. Long-term management of the disease will likely require an integration of care from primary care providers, HIV specialists, and specialists in other therapeutic areas. So a team approach and models of blended care are developing now.”
A greater reliance on HIV specialists was necessary a decade or two ago, when HIV treatment was undergoing rapid changes. Yet, even with the relative stability and slower pace of changes now, there is some concern about a potential shortage of HIV experts.
“The first generation of HIV doctors, those of us who basically learned to treat HIV during our early training, are all aging ourselves,” Dr. Currier says, “so we need to be replaced by younger physicians, and it seems there are fewer people coming out of training who are identifying HIV as their primary focus.”
An important goal for all HIV clinicians will be to address the ways in which current treatment can be undermined by a lack of adherence. Efforts to address ongoing stigma, as well as mental health, substance abuse, and other lifestyle factors that contribute to adherence problems, should go a long way toward improving upon current treatment successes.
“Getting a diagnosis of HIV is still a life-changing event for anyone,” Dr. Currier says, “but the hope that we can offer people today with regard to our ability to effectively treat the disease is remarkable. We still don’t have a cure, and HIV still requires daily treatment, but what I think may still be hard for people to realize, when they initially discover that they have HIV, is that they are going to be OK. They are going to be able live with this disease and do the things in their lives that they wanted to do, and the disease does not have to define them. That is a huge change from how it was in beginning, and we can’t take any of this progress for granted. There are so many forces at play right now in our health care system, and we don’t want to risk losing the progress that we have made.”
Directions in Treatment Research
Antiretroviral therapy (ART) for individuals with HIV infection, which is supported by all treatment guidelines in the U.S., is extraordinarily effective and convenient compared with earlier treatments. Today almost all recommended HIV treatments are a combination of three agents, with a typical treatment regimen consisting of two nucleoside reverse transcriptase inhibitors and a third agent from one of three drug classes: an integrase strand transfer inhibitor (INSTI), a non-nucleoside reverse transcriptase inhibitor (NNRTI), or a protease inhibitor (PI) with an enhancer or booster (Table 1).28
Table 1.
Recommended Initial Regimens for Most People With HIV28
| Selection of a regimen for an individual should be guided by factors such as virological efficacy, toxicity, pill burden, dosing frequency, drug–drug interaction potential, resistance testing results, comorbid conditions, access, and cost. | |
|---|---|
| Generic Formulation | Selected Brand-Name Productsd |
| Dolutegravir/abacavir/lamivudinea,b | Fixed-dose combination: Triumeq (ViiV Healthcare) |
| Dolutegravir plus tenofovir/ emtricitabineb,c | Tivicay (ViiV Healthcare) plus Truvada (Gilead Sciences, with TDF) or Descovy (Gilead Sciences, with TAF) |
| Elvitegravir/cobicistat/tenofovir/ emtricitabinec | Fixed-dose combinations: Genvoya (Gilead Sciences, with TAF) or Stribild (Gilead Sciences, with TDF) |
| Raltegravir plus tenofovir/ emtricitabineb,c | Isentress (Merck) plus Truvada (Gilead Sciences, with TDF) or Descovy (Gilead Sciences, with TAF) |
Only for patients who are HLA-B*5701-negative
Lamivudine may substitute for emtricitabine or vice versa.
Tenofovir alafenamide (TAF) and tenofovir disoproxil fumarate (TDF) are two forms of tenofo-vir approved by the Food and Drug Administration. TAF has fewer bone and kidney toxicities than TDF, while TDF is associated with lower lipid levels. Safety, cost, and access are among the factors to consider when choosing between these drugs.
Individual components of these regimens are available as branded and in some cases generic products.

Paul E. Sax, MD
“All of the recommended regimens can suppress the virus to below the limits of detection—that is, if people take them,” says Paul E. Sax, MD, Clinical Director of the HIV Program and Division of Infectious Diseases at Brigham and Women’s Hospital and Professor of Medicine at Harvard Medical School. “So, the selection of an initial regimen is really based on three things: a treatment’s side effect profile, its efficacy in certain settings—for example, in patients with high viral loads, or in those with low CD4 cell counts—and third, the likelihood that an individual will develop resistance, if in fact he or she doesn’t successfully adhere to the treatment. Taking all of these factors into consideration, the choice that makes the most sense in 2017 is one that includes an integrase strand transfer inhibitor, or INSTI. Integrase-based therapies have become dominant in initial therapy in the U.S. There also has been some refinement of NRTIs—so, even though there are many treatments available, we’ve narrowed them down to just a few that we use most commonly.”
Once a three-drug regimen has been selected, the need for modification of treatment is typically minimal, Dr. Sax notes. The efficacy of current treatments has been so remarkable that resistance to treatment is far less of a problem than it was even a decade ago.
“A small number of people will experience side effects in response to an initial regimen,” Dr. Sax says. “For example, the best current regimens typically include dolutegravir, but roughly one in 20 people who takes dolutegravir will experience insomnia or headache, and for that reason will have to switch to something else. Side effects rather than efficacy are now the primary reasons for switching. Even in very difficult-to-treat populations, efficacy is outstanding for most of these drugs.”
Although resistance to HIV treatments has become much less of a problem than in the past, with only a very small number of people now experiencing virological failure due to resistance, research examining investigational HIV treatments with new mechanisms of action continues. Among new treatments under development (Table 2), the attachment inhibitor fostemsavir, which prevents attachment of the virus to the CD4+ T cell and subsequent entry into the cell, is one example.
Table 2.
Selected Investigational Treatments for HIV Infection
| Agent (Manufacturer/Sponsor) | Description/Mechanism of Action | Indications/Comments | Status |
|---|---|---|---|
| Albuvirtide (Frontier Biotechnologies Co., Ltd.) | Fusion inhibitor; a synthetic peptide that works by binding to the HIV gp41 envelope protein | Developed primarily for China’s national Free Antiretroviral Treatment Program; once-weekly infusion | Phase 3 |
| Bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg (Gilead Sciences) | Bictegravir, a novel INSTI, inhibits strand transfer of viral DNA into host genome and thus prevents HIV-1 replication. In vitro, it has potent antiviral activity against HIV-2 and subtypes of wild-type HIV-1. Has shown synergistic effects in combination with other ARVs, including the dual-NRTI backbone of tenofovir alafenamide and emtricitabine, as well as darunavir | Does not require boosting and can be taken with or without food | NDA filed (target action date, February 12, 2018) |
| Cabotegravir (ViiV Healthcare) | INSTI; an analogue of dolutegravir that prevents viral DNA integration into the host genome and inhibits HIV replication | Being developed for both HIV treatment and HIV prevention | Phase 3 |
| Darunavir 800 mg, cobicistat 150 mg, emtricitabine 200 mg, and tenofovir alafenamide 10 mg (Janssen) | First protease inhibitor-based single-tablet regimen. Darunavir is a protease inhibitor, cobicistat is a pharmacokinetic enhancer, and emtricitabine and tenofovir alafenamide are NRTIs | High barrier to resistance (due to darunavir/ cobicistat), with potentially less renal and bone toxicity compared with regimens that include TDF; potential gastrointestinal adverse effects; potential drug interactions secondary to the cobicistat component | NDA filed |
| Dolutegravir plus lamivudine (ViiV Healthcare) | Dolutegravir is a potent INSTI; lamivudine is a nucleoside analogue | Reported safe and effective as maintenance therapy at Conference on Retroviruses and Opportunistic Infections 2017 | Phase 3 |
| Doravirine (Merck) | Novel NNRTI with in vitro activity against wild-type HIV-1 and common NNRTI- resistant mutant viruses. Resistance to doravirine has been observed with several NNRTI-resistant mutations, including Y188L | Once-daily use being evaluated individually or in a fixed-dose combination with generic lamivudine and TDF. The combination is anticipated to cost less than similar combinations containing fewer generic components | Phase 3 |
| Fostemsavir (ViiV Healthcare) | A prodrug of temsavir; a first-in-class attachment inhibitor that binds directly to HIV-1 gp120, preventing initial viral attachment and entry into host CD4+ T cells | For patients with multidrug-resistant HIV | Phase 3 |
| Ibalizumab (TaiMed Biologics and Theratechnologies) | Monoclonal antibody that blocks initial HIV entry into cells by attaching to CD4 receptors | Biologic developed for the treatment of cross-class/multidrug-resistant HIV. Will require intravenous infusions (800 mg) every two weeks | BLA filed; approval anticipated in first half of 2018 |
| PRO140 (CytoDyn, Inc.) | HIV-1 entry inhibitor; humanized IgG4 monoclonal antibody inhibits CCR5-mediated HIV-1 viral entry | Once-weekly subcutaneous injection for maintenance of viral suppression | Phase 3 |
| Rilpivirine (Janssen) and cabotegravir (ViiV Healthcare) | Rilpivirine is an NNRTI; cabotegravir is an INSTI | Combination of two long-acting formulations for intramuscular injection. Phase 3 studies are evaluating dosing every four weeks | Phase 3 |
ARV = antiretroviral; BLA = biologics license application; HIV = human immunodeficiency virus; INSTI = integrase strand transfer inhibitor; NDA = new drug application; NNRTI = non-nucleoside reverse transcriptase inhibitor; NRTI = nucleotide reverse transcriptase inhibitor; TDF = tenofovir disoproxil fumarate. Sources: National Institutes of Health (via https://aidsinfo.nih.gov); manufacturers’ websites; meeting abstracts.
“Drugs with new mechanisms of action will continue to be of value, because having more options for patients is always a good thing,” Dr. Sax says. “But after the approval of bictegravir/emtricitabine/tenofovir AF in early 2018, the future of HIV treatment is not going to be about developing just another single pill that you can take once a day. The goal will be to develop an intervention that goes beyond that. That might be a long-acting preparation that can be taken once a month or once a quarter, or it may be an injectable or implantable preparation. To be of significant value it will have to be something completely different from what we have now.”
A two-drug, long-acting injectable combination of rilpivirine (Janssen) and cabotegravir (ViiV Healthcare), which may be taken every four or eight weeks, is one promising treatment under investigation that may offer hope for improved adherence. The Ninth International AIDS Society (IAS) Conference on HIV Science, which took place in Paris in July 2017, featured the results of the phase 2b Long-Acting Antiretroviral Treatment Enabling (LATTE-2) trial,29 which is investigating this combination. Early results showed that among individuals who received intramuscular injections every eight weeks, 94% (n = 108) achieved viral suppression.29
Several two-drug combinations are under investigation and have appeal because of their potential for reducing drug toxicity and limiting the cost of lifelong treatment. Such regimens will likely be used as maintenance therapy, in individuals whose viral load is already undetectable, and who require a less intensive regimen to maintain suppression of the virus.
“One two-drug combination that was just approved was the single tablet of dolutegravir plus rilpivirine [Juluca, ViiV Healthcare],” 30 Dr. Sax says, “not as initial therapy but for patients already virologically suppressed and with no history of resistance or treatment failure. An advantage of this combination is its very small tablet size, which may help to improve adherence in individuals who have difficulty swallowing larger pills. A disadvantage is that rilpivirine as an oral treatment is somewhat less potent than others, and also requires that people take it with food fairly meticulously—otherwise it isn’t absorbed well. It’s not a treatment that is going to transform HIV therapy, but it will be a welcome option for certain stable patients who might benefit from not being on nucleoside reverse transcriptase inhibitors, specifically tenofovir or abacavir.”
A monotherapy, which might be expected to further reduce drug toxicity and possibly cost, does not appear to be on the horizon. While dolutegravir briefly seemed to be a promising candidate for monotherapy, studies presented at CROI demonstrated dolutegravir’s vulnerability to virological failure and to the development of resistance when used as monotherapy.31
“A single-drug approach still might be a possibility, if we can identify an extremely potent compound. We got a glimpse of that possibility earlier this year,” Dr. Sax says, “during the first presentation of research on a capsid inhibitor—a drug with a novel mechanism that was extraordinarily potent in vitro, more potent than any antiviral. But a monotherapy is unlikely to be available any time soon.”
While a vaccine also is not expected to emerge within the next couple of years, Dr. Sax points to two studies that are particularly encouraging. The HIV vaccine study HVTN 702 is testing whether an experimental vaccine regimen will safely prevent HIV infection among adults in South Africa. HVTN 702 is a placebo-controlled trial that aims to enroll 5,400 men and women in South Africa, where it is estimated that more than 1,000 people become infected with HIV each day.32 Also noteworthy are results from an early stage, phase 1/2a APPROACH study for an investigational HIV vaccine regimen being developed by Janssen. Presented at the Ninth IAS Conference in 2017, early data showed that the “mosaic”-based vaccine regimen (“mosaic” vaccines are designed to induce immunological responses against a wide variety of HIV subtypes) appeared to be well tolerated and elicited HIV-1 antibody responses in 100% of healthy volunteers (n = 393).33
In the realm of cure research, where a promising intervention has yet to emerge, an important goal should be to determine exactly how safe and how effective an HIV cure would have to be in order to represent an acceptable alternative to current ART options. In an article published in February 2017,34 Dr. Sax and coauthor Kenneth Freedberg, MD, argued that an estimation of the clinical impact and cost-effectiveness of a potential cure should take place before its development to establish realistic goals for researchers and policy-makers. Such planning may help researchers avoid pursuing impractical strategies. For example, the method leading to the only known HIV cure35 represents a less than promising path of research.
“The individual who was cured had leukemia, and he happened to receive a stem cell transplant from a person who had a genetic mutation called CCR5-delta 32, which means the cells the patient acquired were highly resistant to getting infected themselves. The patient not only received a stem cell transplant, which markedly reduced his HIV reservoir, but he received cells that were not easily infected by HIV. Ultimately, this success was the result of an extraordinary experiment—tantalizing, but not in any sense broadly applicable to HIV cure research. The risks associated with stem cell transplantation are far too great,” Dr. Sax explains.
Dr. Sax and Dr. Freedberg incorporated hypothetical cure strategies such as gene therapy, chemotherapy, and stem cell transplantation into an analysis examining the cost, efficacy, and toxicity of potential cures. Even without access to real research data, they were able to conclude that current treatments have already set a very high bar for any potential cure.
“Because it is now possible for a person with HIV to remain alive and healthy by taking one or two pills a day,” Dr. Sax says, “it is not ethically sound to offer an HIV cure if there is a 1% chance that the patient will die from it. Some potential cure strategies are scientifically interesting but not justifiable in terms of their risk. In our effort to cure HIV we have to make sure that we are not developing a treatment that is more toxic than the disease that we are controlling.”
Ending the Epidemic in the U.S
Because ART has significantly reduced AIDS-related morbidity and mortality and greatly improved long-term outcomes for people with HIV, many outside of the field may be surprised to learn that experts still widely refer to the disease as an epidemic, even within the United States. As long as U.S. rates of new diagnoses continue to be significant, calling the disease an epidemic will remain appropriate, as will restoring to HIV/AIDS the priority status it once held, according to Melanie Thompson, MD, a member of the antiretroviral guidelines panels of the U.S. Department of Health and Human Services and the International Antiviral Society–USA, who also is founder and principal investigator of the AIDS Research Consortium of Atlanta.
“Even without a cure or a vaccine, we know how to end AIDS in America,” says Dr. Thompson, who serves on the Executive Committee of Fulton County’s Task Force on HIV/AIDS in Georgia and chairs the HIV Medicine Association of the Infectious Diseases Society of America. “The fight against HIV is falling victim to public and political apathy and to a fundamental lack of understanding with regard to what exactly is needed to end the epidemic. We also are at risk of erosion of our health care system and funding for critical public programs. If we don’t step up and make this a key issue again we will lose ground on all the progress we have made. We are at a tipping point.”
The Fulton County Task Force on HIV/AIDS was established amid community advocacy in response to the county’s alarmingly high rate of new infections. The Board of Commissioners and the HIV community joined to establish a broad community collaboration and develop a plan of action, incorporating wisdom gained from similar efforts in major cities such as New York and San Francisco. Dr. Thompson is Executive Editor of the Strategy to End AIDS in Fulton County,36 the first comprehensive jurisdictional plan of its kind in the south. Such blueprints will continue to be essential in meeting the needs of individuals with HIV in the U.S., whose numbers continue to increase steadily—and not just due to new infections.
“The increase in numbers every year is in some sense a good thing, because we are keeping people alive longer,” Dr. Thompson says. “HIV infection once meant inevitable death, but with effective treatments that has changed dramatically. Yet we now have a steadily increasing number of people needing services, and that is really putting a stress on our system, not just in Fulton County but nationwide.”
Task Force Strategies
Many of the recommendations by Fulton County’s task force overlap with those of the NHAS, while others are more aggressive. The first recommendation is to expand HIV testing. While the CDC estimates that about 15% of infected people in the U.S. are unaware of their status,7 Dr. Thompson says that number is likely to be significantly higher in some areas. For example, she estimates that some populations in Fulton County, such as African-American gay and bisexual men, may have undiagnosed HIV rates of close to 20%, and that as many as 3,000 people in Fulton County may have HIV without being aware of their status. Therefore, expanding HIV testing is a critical goal, beginning with greater efforts to implement opt-out testing.

Melanie Thompson, MD
“In 2006, CDC released guidance that recommended routine opt-out testing in health care settings,” Dr. Thompson says. “Opt out means that you don’t have to go through the arduous process of giving people written informed consent to do HIV testing. People are told they will have an HIV test done unless they say they don’t want it. That is very different and far more effective than requiring someone to opt in. HIV testing should be a routine part of medical care, much like having your cholesterol checked.”
Although the CDC recommended opt-out testing more than a decade ago, Dr. Thompson says that the uptake in clinical settings has been poor. The task force recommended that Fulton County institute routine opt-out testing in all ambulatory care facilities, neighborhood clinics, and all facilities, such as hospitals, for which it provides funding. A key goal is to make sure that people are being diagnosed at an earlier stage of infection.
“We know that in our area as many as a third of people may already have AIDS by the time that they are diagnosed. That is tragic, and it’s unnecessary. We want to ensure that people are diagnosed much earlier, to keep them healthy and prevent transmission of HIV to others,” Dr. Thompson says.
Just as important as testing and early diagnosis are efforts to ensure that immediately after diagnosis people with HIV have access to comprehensive, uninterrupted clinical care.
“We know that 90% of new HIV infections can be traced to people who are diagnosed but not receiving care, and that about 60% can be traced to people who have been in care and subsequently fallen out of care, and therefore don’t have suppressed virus,” Dr. Thompson says. “We need to be sure that it is easier for all people with HIV to get into treatment, and to stay on their HIV medications. When people are on effective HIV medication, with an undetectable level of virus, they do not spread the virus to others—that’s one way we can end transmission of the virus.”
The task force recognized the importance of improving on the federal goal of getting people into treatment within 30 days of diagnosis.
“When we listened to members of the community, we discovered that it often requires several visits to get someone into care, and during that period we frequently lose people,” Dr. Thompson says. “We decided that we have to be able to get people into medical care, not just to an administrative visit, within three days of diagnosis or presentation for reengagement. Other places in the U.S. and around the world are also beginning to do this now. San Francisco has been implementing a program with the goal of getting people into care and on HIV medication on the same day.”
In older models of care, Dr. Thompson adds, people often saw a social worker first, and had to provide identification and demonstrate that they qualified to receive treatment. It was often two to four visits, or weeks later, before they saw a medical provider, and many never came back.
“Our goal is to take care of people first. Documentation might be important, but everything doesn’t have to be in place on the first day. Our aim is to get people started on treatment and then pull the documentation together in the subsequent 30 days,” Dr. Thompson says.
The task force is also following through on other recommendations, including increasing HIV education and awareness, beginning with a successful collaboration with Atlanta public schools to introduce a comprehensive, scientifically accurate sexual education curriculum. And the task force is committed to reforming outdated criminalization laws that lead to prosecution for failing to disclose one’s HIV status. Fighting barriers to providing PrEP to populations that need it most and reducing stigma are two other important goals.
The Ryan White HIV/AIDS Program, which Dr. Thompson says is the most effective HIV care system in the United States and a good model for initiating care and retaining patients, is facing a potential loss of funding. Meanwhile, task forces in Atlanta and other major cities are doing their best to operate with what Dr. Thompson calls “a void of leadership at the White House.”
“Ryan White was created as a public health intervention, and it’s essential that it continue to operate with a robust budget if we want to end the epidemic in this country,” says Dr. Thompson, who also stresses that the Patient Protection and Affordable Care Act has dramatically improved the lives of those with HIV, many of whom were previously uninsurable. “HIV is not hypertension or Alzheimer’s disease, it is a communicable infectious disease that kills people. Many in this country who have heard about effective HIV treatments and increased lifespan fail to understand the complexities of HIV care and the extent to which it is fragile. If we are going to end this epidemic, we have to get people tested and into care. We have to ensure that people stay in care. And we need to have the appropriate public and private infrastructure to do that. Advocacy is now a part of HIV care”
REFERENCES
- 1.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4(8):e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roxby P. HIV life expectancy ‘near normal’ thanks to new drugs. BBC. May 11, 2017. [Accessed November 28, 2017]. Available at: www.bbc.com/news/health-39872530.
- 3.Preidt R. Life expectancy with HIV nears normal with treatment. CBS News. May 11, 2017. [Accessed November 28, 2017]. Available at: www.cbsnews.com/news/life-expectancy-with-hiv-nears-normal-with-treatment.
- 4.Bonacci RA, Holtgrave DR. U.S. HIV incidence and transmission goals, 2020 and 2025. Am J Prev Med. 2017;53(3):275–281. doi: 10.1016/j.amepre.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Walensky RP, Borre ED, Bekker L-G, et al. Do less harm: evaluating HIV programmatic alternatives in response to cutbacks in foreign aid. Ann Intern Med. 2017;167(9):618–629. doi: 10.7326/M17-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horn T, Sherwood J, Remien RH, et al. Towards an integrated primary and secondary HIV prevention continuum for the United States: a cyclical process model. J Int AIDS Soc. 2016;19(1):21263. doi: 10.7448/IAS.19.1.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. HIV/AIDS. Basic statistics. [Accessed November 27, 2017]. Available at: www.cdc.gov/hiv/basics/statistics.html.
- 8.White House Office of National AIDS Policy. The national HIV/AIDS Strategy: updated to 2020. Jan 31, 2017. [Accessed October 10, 2017]. Available at: www.hiv.gov/federal-response/national-hiv-aids-strategy/nhasupdate.
- 9.UNAIDS. 90–90–90—An ambitious treatment target to help end the AIDS epidemic. Jan 1, 2017. [Accessed November 28, 2017]. Available at: www.unaids.org/en/resources/documents/2014/90-90-90.
- 10.Diverse Elders Coalition. Issue brief: Eight policy recommendations for improving the health and wellness of older adults with HIV. 2014. [Accessed November 28, 2017]. Available at: https://sageusa.org/files/DEC-HIVand-Aging-Policy-Report_web.pdf.
- 11.Currier JS, Havlir DV. CROI 2017: Complications and comorbidities of HIV disease and its treatment. Top Antivir Med. 2017;25(2):77–83. Available at: www.iasusa.org/sites/default/files/tam/25-2-77.pdf. Accessed November 28, 2017. [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Romieu AC, Garg S, Rosenberg ES, et al. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. doi: 10.1136/bmjdrc-2016-000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen L, Smith B, Reich D, et al. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis, and treatment. Nat Rev Neurol. 2016;12(11):662–674. doi: 10.1038/nrneurol.2016.149. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt CM. Kidney disease and HIV. Top Antivir Med. 2017;25(1):13–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez J, Albuquerque A, Falzon L. HIV infection as vascular risk: A systematic review of the literature and meta-analysis. PLoS One. 2017;12(5):e0176686. doi: 10.1371/journal.pone.0176686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Easterbrook PJ, Roberts T, Sands A, Peeling R. Diagnosis of viral hepatitis. Curr Opin HIV AIDS. 2017;12(3):302–314. doi: 10.1097/COH.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu C, Pollock LC, Selwyn PA. HIV-associated complications: a systems-based approach. Am Fam Physician. 2017;96(3):161–169. [PubMed] [Google Scholar]
- 18.Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) About REPRIEVE. [Accessed November 28, 2017]. Available at: www.reprievetrial.org/overview.
- 19.Curno MJ, Rossi S, Hodges-Mameletzis I, et al. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71(2):181–188. doi: 10.1097/QAI.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Pre-exposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. Sep, 2017. [Accessed November 28, 2017]. Available at: https://stacks.cdc.gov/view/cdc/48352.
- 21.Fact sheet: The national HIV/AIDS strategy: updated to 2020. Jul 30, 2015. [Accessed November 28, 2017]. Available at: https://obamawhitehouse.archives.gov/the-press-office/2015/07/30/fact-sheet-national-hivaids-strategyupdated-2020.
- 22.Sheth AN, Rolle CP, Gandhi M. HIV pre-exposure prophylaxis for women. J Virus Erad. 2016;2(3):149–155. [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. HIV among women. [Accessed November 28, 2017]. Available at: www.cdc.gov/hiv/group/gender/women/index.html.
- 24.Adimora AA. Preventing HIV among women—a step forward, but much farther to go. N Engl J Med. 2016;375(22):2195–2196. doi: 10.1056/NEJMe1613661. [DOI] [PubMed] [Google Scholar]
- 25.Barry SM, Mena Lora AJ, Novak RM. Trial, error, and breakthrough: a review of HIV vaccine development. J AIDS Clin Res. 2014 Oct;5:359. doi: 10.4172/2155-6113.1000359. [DOI] [Google Scholar]
- 26.Lawrence D, Kuo L, Church E, et al. Highlights from the third biennial strategies for an HIV cure meeting. J Virus Erad. 2017;3(1):69–76. [PMC free article] [PubMed] [Google Scholar]
- 27.Garner S, Rennie S, Ananworanich J, et al. Interrupting antiretroviral treatment in HIV cure research: scientific and ethical considerations. J Virus Erad. 2017;3(2):82–84. [PMC free article] [PubMed] [Google Scholar]
- 28.Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Oct 17, 2017. [Accessed November 28, 2017]. Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0.
- 29.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration. FDA approves first two-drug regimen for certain patients with HIV. Nov 21, 2017. [Accessed November 28, 2017]. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm586305.htm?utm_campaign=11212017_FDA%20approves%20two%20drug%20regimen%20for%20HIV&utm_medium=email&utm_source=Eloqua.
- 31.Wijting I, Rokx C, Boucher C, van Kampen J. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial [published online October 26, 2017] Lancet HIV. doi: 10.1016/S2352-3018(17)30152-2. pii: S2352-3018(17)30152-2. [DOI] [PubMed] [Google Scholar]
- 32.ClinicalTrials.gov. Pivotal phase 2b/3 ALVAC/bivalent gp120/MF59 HIV vaccine prevention safety and efficacy study in South Africa (HVTN702). NCT02968849. Dec 29, 2016. [Accessed November 28, 2017]. Available at: https://clinicaltrials.gov/show/NCT02968849.
- 33.Center for Virology and Vaccine Research. Investigational preventative HIV vaccine well-tolerated, elicited antibody responses in early clinical trials. Jul 24, 2017. [Accessed November 28, 2017]. Available at: http://cvvr.hms.harvard.edu/blog/http/wwwbidmcorg/news/prlandingpage/2017/july/barouch-investigational-preventative-hivaspx.
- 34.Freedberg KA, Sax PE. Improving on effective antiretroviral therapy: how good will a cure have to be? J Med Ethics. 2017;43(2):71–73. doi: 10.1136/medethics-2016-103907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 36.Fulton County HIV Task Force. Strategy to end AIDS in Fulton County. [Accessed November 28, 2017]. Available at: www.fultoncountyga.gov/hivtf-strategy-to-end-aids.