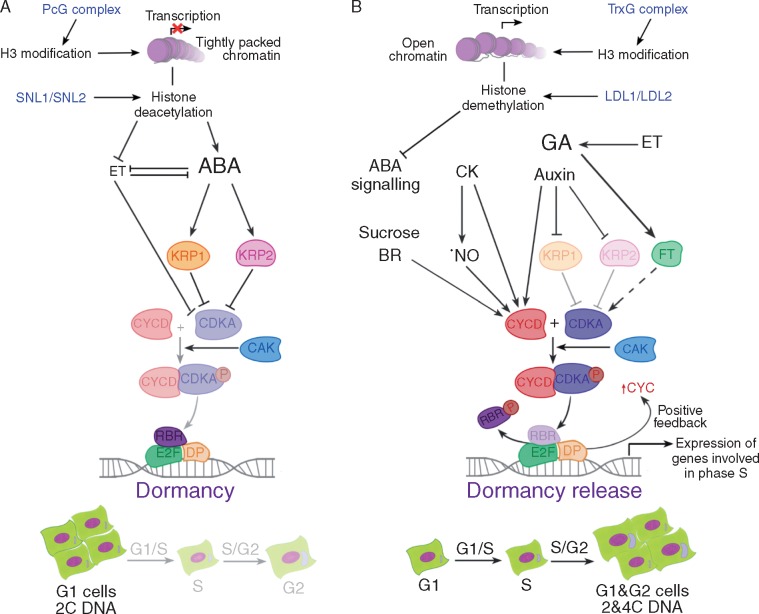
Fig. 2.
Regulation of hormone control of the G1/S transition during dormancy by chromatin regulators. (A) In the dormant state most of the cells have 2C DNA, so it is considered that there is strong regulation of the G1/S transition. RBR mediates the repression of genes involved in the S phase. Abscisic acid (ABA) induces the activity of CDKA inhibitors, KRP1 (ICK1) and KRP2 (ICK2), inhibiting G1/S transition. Although it is suggested that ethylene (ET) and ABA are antagonistic, the signalling cascade induced by ET has also been suggested to repress CDKA activity. The histone deacetylation mediated by SNL1 and SNL2 is suggested to promote this ABA–ethylene antagonism. This modification would be relevant in the dormant state. (B) Histone demethylation, mediated by LDL1 and LDL2, would have implications for dormancy release by reducing ABA signalling (by repressing the expression of ABA2, ABI3 and ABI5). Cytokinins (CK) induce CYCD both by themselves and by inducing nitric oxide (•NO) accumulation. GA represses the CDK inhibitors and promotes CYC and FT, which is suggested to induce CDK. Moreover, ET induces gibberellic acid (GA) signalling. In such a situation the complex CYCD/CDKA is able to phosphorylate RBR, allowing the expression of genes involved in S phase. Hence the non-dormant state is characterized by the presence of a greater G1:G2 ratio. CAK, CDK-activating kinases; CDK, cyclin dependent kinases; CYC, cyclins; LDL, lysine-specific demethylase like; RBR, retinoblastoma related protein; SNL, SIN3-like.