ABSTRACT
We analyzed 428 femoral metastases initially treated with radiotherapy between 2002 and 2011 to clarify the clinical details of post-irradiation fractures of femoral metastasis. Patients included 161 men and 167 women, with a mean age of 62 years. Fracture incidence, fracture site, fracture risk based on X-ray images before radiotherapy, and interval from completion of radiotherapy to fracture occurrence were assessed. In addition, 24 pathological specimens obtained during 27 surgeries for these fractures were examined. Fractures occurred in 7.7% of 428 femoral metastases (total 33: 28 actual fractures and five virtual fractures with progressive pain and bone destruction). The fracture rate was 7.8% in the proximal femur and 1.5% in the shaft (P = 0.001). Fractures occurred a median of 4.4 months after radiotherapy, with 39.4% occurring within 3 months and 63.6% within 6 months. Among femurs with high fracture risk according to Harrington’s criteria or Mirels’ score, the fracture rate was 13.9% and 11.8%, respectively. Viable tumor cells were detected in all five patients with painful virtual fracture, in 85.7% of femurs with actual fractures that occurred within 3 months, and in only 25.0% of actual fractures occurring after 3 months. Post-irradiation fractures of femoral metastasis most frequently occurred within 3 months after radiotherapy, and were more common in the peritrochanteric area than in the shaft. Radiological evidence of impending fracture did not correlate with a high fracture rate. Actual fractures occurring after more than 3 months were likely caused by post-irradiation fragility of the femur, without viable tumor cells.
Keywords: bone metastasis, femur, fracture, radiation therapy
INTRODUCTION
The femur is the third most common site for bone metastasis requiring treatment, after the spine and the pelvis [1, 2]. When metastasis develops in the femur, the initial symptom is pain, with pathological fracture possible with disease progression. Palliative radiotherapy for painful bone metastasis is well established and frequently performed, with a total dose of 30 Gy delivered in 10 fractions considered a standard schedule [3, 4]. It has been reported that pain relief is experienced by ~47% of patients who undergo radiation therapy for femoral metastasis, and that fractures are avoided in >80% of cases, even in those with signs of impending fracture [5, 6].
Unfortunately, some patients sustain pathologic fracture even after radiation therapy; various rates of pathologic fracture have been reported. Keene et al. reported in 1984 that post-irradiation fracture of femoral metastasis (PIFF) occurred in 5 of 56 patients (8.9%) [7]. In 2004 Linden et al. reported that PIFF developed in 12.7% of 110 femurs [6, 8]. Harada et al. reported that 13% of 84 lesions eventually required surgery [5]. Even with the availability of bone-modifying agents in the last 10 years, a fracture rate of 25% has been reported [9]. However, these reports did not include large numbers of patients, and none of the reports described the histology of the fracture sites. The purpose of this study was to analyze a large patient series to clarify the incidence and detailed clinical characteristics of PIFF, including the histology of the fracture site, as well as the relationship between PIFF and radiological criteria of impending fracture.
MATERIALS AND METHODS
We treated 501 femoral metastases at our hospital between 2002 and 2011. Among these, 73 femurs (28 impending and 45 actual fractures) were initially treated with surgery. This investigation was carried out in the remaining 428 femurs in 328 patients initially treated with radiation therapy. There were 203 affected femurs in 161 male patients and 225 affected femurs in 167 female patients, with an average patient age of 62 years (range, 30–92 years). The common primary lesions included breast cancer: 125 femurs (29.2%); lung cancer: 101 femurs (23.6%); prostate cancer: 46 femurs (10.7%); gastrointestinal tract cancer: 38 femurs (8.8%); hepatocellular carcinoma (HCC): 14 femurs (3.2%); and other cancer: 104 femurs (24.3%). The average irradiation dose was 30.8 Gy (range, 8 Gy in a single fraction to 50.4 Gy in 28 fractions) (Table 1).
Table 1.
Baseline characteristics of all patients
| Characteristic | Value (range) | Femurs (number of patients) | % |
|---|---|---|---|
| Age (mean, years) | 62 (30–92) | ||
| Gender | |||
| Male | 203 (161) | 47.4 | |
| Female | 225 (167) | 52.6 | |
| Radiation dose (Gy) | |||
| 8–27 | 90 | 21 | |
| 30–39 | 325 | 75.9 | |
| 40–50.4 | 13 | 3 | |
| Mean | 30.8 (8–50.4) | ||
| Radiation field | |||
| Proximal only | 230 | 53.7 | |
| Proximal and distal | 153 | 35.7 | |
| Distal only | 45 | 10.5 | |
| Primary site | |||
| Breast | 125 (82) | 29.2 | |
| Lung | 101 (89) | 23.6 | |
| Prostate | 46 (34) | 10.7 | |
| Esophagus, stomach, intestine, colon, rectum | 38 (29) | 8.9 | |
| Unknown primary origin | 28 (24) | 6.5 | |
| Kidney | 14 (12) | 3.3 | |
| Liver | 14 (10) | 3.3 | |
| Myeloma | 10 (6) | 2.3 | |
| Thyroid | 7 (6) | 1.6 | |
| Others | 45 (36) | 10.5 | |
The treatment of femoral metastasis, including radiation planning and surgical indication, was determined at bone metastasis board meetings at our hospital. At our institution, apparent impending fractures with pain were treated surgically unless the patient’s general condition was poor. In patients in whom surgery was not possible because of poor general condition, in those with little bone destruction and a primary aim of pain relief, and for metastasis with apparent bone destruction from cancers sensitive to radiation therapy, radiation therapy was performed. Harrington’s criteria of impending fracture (cortical bone destruction ≥50%, lesion ≥2.5 cm, avulsion fracture of the lesser trochanter) were used to determine the risk of impending fracture [10]. In patients with apparent bone destruction, 3 months of partial weight-bearing with single or double crutches was prescribed. All patients provided informed consent for each treatment.
We retrospectively reviewed patient background, total radiation dose, irradiated area, fracture site, Mirels’ score, and survival period for all patients [11]. From these data, the incidence of PIFF, the relationship between the irradiated area and the PIFF site, the interval from the completion of radiotherapy to PIFF occurrence, the relationship between radiological fracture risk and PIFF event, and the relationship between patient background and PIFF were investigated. To evaluate the relationship between the irradiated area and the fracture site, the femur was divided into two regions: proximal (femoral head, neck and peritrochanteric area) and distal (diaphysis and distal condylar segment). This simple division was chosen because radiation therapy for the femur usually does not target a narrow field such as the femoral head alone, but normally targets a wide field such as the proximal third of the affected femur, including the femoral head and subtrochanteric area. Radiographic imaging before treatment was reviewed and scored separately by two experienced orthopedic surgeons. If there was discrepancy in scoring, the observers re-examined the imaging and discussed their observations to reach a consensus. Background factors, including age (≥62 years or <62 years), irradiation dose (≥30 Gy or <30 Gy) and zoledronic acid administration (regular 4-week interval administration or not) were investigated with a χ2 test to determine their association with fracture occurrence. We divided the study population into groups according to irradiation dose (≥30 Gy or <30 Gy) for two reasons. First, a total dose of ≥30 Gy delivered in 10–20 fractions was shown to achieve better local control in the spine than short-course radiotherapy, and we wanted to determine whether this difference could be applied to femoral metastasis [12]. In addition, a total dose of 30 Gy delivered in 10 fractions was the most commonly used schedule for painful bone metastasis, and many radiation oncologists remained reluctant to use short-course radiation therapy [4]. Values of P < 0.05 were considered significant. Survival analysis was performed with Kaplan–Meier methods. Furthermore, histopathology of the fracture site was performed for those patients who underwent surgery for PIFF.
This study was carried out with the approval of the Institutional Review Board at our institution. The treatment policy for each patient was discussed in a multidisciplinary bone metastasis board meeting (including radiation oncologists and orthopedic surgeons) to determine the appropriate therapeutic approach. All data were obtained in routine clinics, and all patients gave their informed consent for each treatment.
RESULTS
Incidence of post-irradiation fractures of femoral metastasis
PIFF occurred in 33 (7.7%) of 428 femurs. Among these, 28 (6.5%) were actual fractures and five (1.2%) were virtual fractures with progressive pain and bone destruction requiring surgery. The average irradiation dose of patients with PIFF was 34.0 Gy (range, 20 Gy in five fractions to 39 Gy in 13 fractions). Among the 395 femurs without PIFF, the average dose was 30.7 Gy (range, 8 Gy in a single fraction to 50.4 Gy in 28 fractions). The common primary cancers in patients with fracture were breast cancer (30.3%), lung cancer (27.3%) and prostate cancer (6.1%). These three cancers accounted for ~70% of fractures, which was not substantially different from the general population of this study (Tables 2, 3).
Table 2.
Characteristics of the 33 patients with fractures
| Characteristic | Value (range) | In fractured femurs (%) |
|---|---|---|
| Age (mean, years) | 64 (37–87) | |
| Gender | ||
| Male | 18 | 54.5 |
| Female | 15 | 45.5 |
| Radiation dose (Gy) | ||
| 20 | 3 | 9.1 |
| 30–39 | 30 | 90.1 |
| mean | 34 (20–39) | |
| Radiation field | ||
| Proximal only | 21 | 63.6 |
| Proximal and distal | 9 | 27.3 |
| Distal only | 3 | 9.1 |
| Primary tumor | ||
| Breast | 10 | 30.3 |
| Lung | 9 | 27.3 |
| Esophagus, colon | 3 | 9.1 |
| Prostate | 2 | 6.1 |
| Unknown origin | 2 | 6.1 |
| Liver | 2 | 6.1 |
| Others | 5 | 15.2 |
Table 3.
Details of the 33 patients who sustained a post-irradiation fracture of femoral metastasis (PIFF)
| Case | Gender | Primary site | Bone destruction | Dose, (Gy) | Time to fracture after RT (months) | Actual fracture or virtual fracture | Pathological examination |
|---|---|---|---|---|---|---|---|
| 1 | M | Lung | Lytic | 20 | 0.0 | A | TC+ |
| 2 | F | Livera | Lytic | 39 | 0.1 | A | NP |
| 3 | M | Bladder | Lytic | 30 | 0.1 | A | TC− |
| 4 | M | Esophagus | Lytic | 39 | 0.2 | V | TC+ |
| 5 | M | Liverb | Lytic | 20 | 0.3 | A | NP |
| 6 | M | Esophagus | Lytic | 39 | 0.4 | A | TC+ |
| 7 | F | Breast | Lytic | 30 | 0.6 | A | TC+ |
| 8 | F | Breast | Mixed | 39 | 0.9 | A | NP |
| 9 | M | Lung | Lytic | 30 | 0.9 | A | NP |
| 10 | M | Lung | Lytic | 20 | 1.1 | A | NP |
| 11 | F | Breast | Mixed | 30 | 1.3 | A | TC+ |
| 12 | F | Breast | Mixed | 30 | 1.4 | A | TC+ |
| 13 | M | Livera | Lytic | 39 | 2.7 | A | TC+ |
| 14 | M | Thyroid | Lytic | 39 | 3.1 | V | TC+ |
| 15 | M | Liverb | Lytic | 39 | 3.4 | A | TC+ |
| 16 | F | Myeloma | Lytic | 39 | 4.0 | V | TC− |
| 17 | M | Prostate | Mixed | 30 | 4.4 | A | TC− |
| 18 | M | Lung | Mixed | 39 | 4.7 | A | NP |
| 19 | M | Lung | Lytic | 39 | 5.7 | A | NP |
| 20 | F | Breast | Lytic | 39 | 5.7 | A | TC− |
| 21 | F | Lung | Lytic | 39 | 5.8 | A | TC− |
| 22 | F | Colon | Lytic | 30 | 7.2 | A | TC− |
| 23 | M | Lung | Lytic | 30 | 7.5 | A | TC− |
| 24 | M | Lung | Lytic | 30 | 7.6 | A | NP |
| 25 | M | Rectum | Lytic | 30 | 7.7 | V | TC+ |
| 26 | F | Breast | Lytic | 39 | 8.2 | A | NP |
| 27 | F | Breast | Lytic | 36 | 9.4 | A | TC− |
| 28 | M | Prostate | Lytic | 30 | 10.4 | A | TC+ |
| 29 | M | Prostate | Lytic | 39 | 10.5 | A | TC− |
| 30 | F | Lung | Lytic | 39 | 14.4 | V | TC+ |
| 31 | F | Breast | Mixed | 39 | 15.4 | A | TC+ |
| 32 | F | Breast | Lytic | 30 | 16.2 | A | TC− |
| 33 | F | Breast | Lytic | 39 | 21.0 | A | TC+ |
M = male, F = female. TC+ = tumor cells present, TC− = tumor cells not present, NP = histopathology not performed, A = actual fracture, V = virtual fracture. aHepatocellular carcinoma. bCholangiocellular carcinoma.
Location of post-irradiation fractures of femoral metastasis
PIFF occurred in the femoral head and neck region in nine femurs, in the peritrochanteric region in 20, in the shaft in three, and in another location in 1. As for the relationship between the irradiated area and the incidence of fractures, fractures in the proximal femur occurred in 30 (7.8%) of 383 femurs in which radiation therapy included the proximal femur. In contrast, shaft fracture occurred in 3 (1.5%) of 198 femurs in which radiation therapy included the distal femur (diaphysis to condylar segment) (Table 2). PIFF occurred significantly more frequently in the proximal than the distal femur (χ2 test, P = 0.001) (Table 4).
Table 4.
Relationship between the occurrence of femoral fractures after radiotherapy and background characteristics (χ2 test)
| With fracture | No fracture | P value | |
|---|---|---|---|
| Radiation field | 0.001 | ||
| Proximal | 30a | 353b | |
| Shaft | 3a | 195b | |
| Age | 0.28 | ||
| <62 | 13a | 194b | |
| ≥62 | 20a | 201b | |
| Radiation dose | 0.08 | ||
| <30 Gy | 3a | 87b | |
| ≥30 Gy | 30a | 308b | |
| Zoledronic acid administration | 0.40 | ||
| Regular | 11a | 105b | |
| Irregular or none | 22a | 290b |
aNumber of femurs with fractures. bNumber of femurs without fractures
Interval from radiation to post-irradiation fractures of femoral metastasis
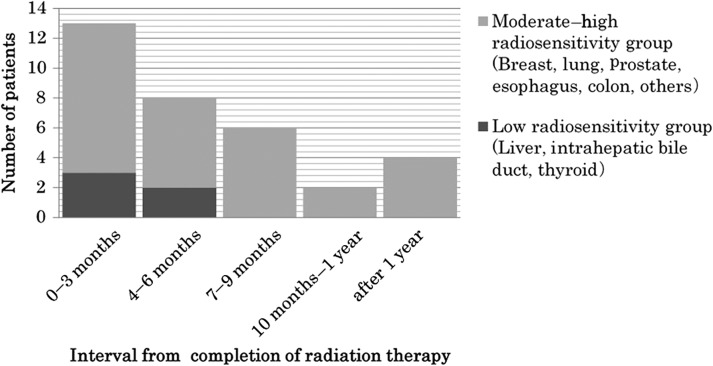
The median interval from the completion of radiotherapy to PIFF was 4.4 months. PIFF occurred most frequently within 3 months after radiotherapy (13 femurs; 39.4%), followed by 4–6 months (eight femurs; 24.2%), 7–9 months (six femurs; 18.2%) and 10–12 months after radiotherapy (two femurs; 6.1%). Twenty-nine (87.8%) of 33 PIFF occurred within 1 year (Fig. 1). However, four (12.1%) fractures (three actual fractures and one virtual fracture) occurred after 1 year, one each at 14, 15, 16 and 21 months (Fig. 1). All three actual fractures occurred without traumatic event. These patients complained of no pain or only slight pain, and follow-up X-ray images showed no progressive bone destruction. In the fourth patient, osteolytic change and pain progressed, even after 39 Gy of radiotherapy, and was attributed to a virtual fracture after 14 months.
Fig. 1.
Time to fracture after radiotherapy for femoral metastasis. Post-irradiation fractures occurred most frequently within the first 3 months (39.4%) after irradiation, and became less frequent over time.
Thyroid cancer, HCC, cholangiocellular carcinoma, and renal cell carcinoma are characterized by soft-tissue expansion, hypervascularity, and low sensitivity to irradiation [13–19]. It is reportedly difficult to achieve local control with radiation therapy for these mass-type bone metastases [20]. There were five cases of these cancers with low radiation sensitivity among the patients with PIFFs. Three of these five fractured within 3 months, and the remaining two fractured within 4 months (Fig. 1, Table 3).
Radiographic impending fracture and post-irradiation fractures of femoral metastasis
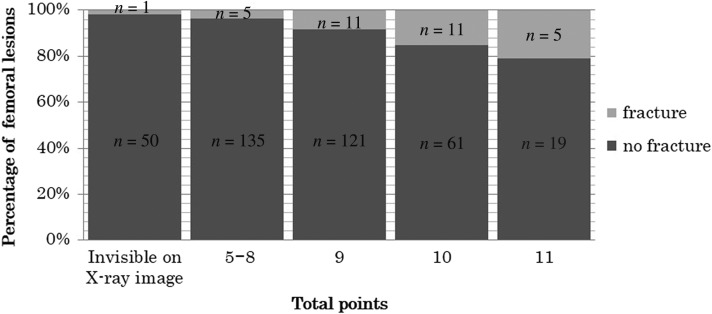
For nine femurs, radiation therapy was performed without X-ray images; in the remaining 419 femurs, fracture risk was evaluable on plain X-ray images. According to Harrington’s criteria, 137 femurs were evaluated as likely to be subject to impending fracture and the remaining 282 femurs as not likely to be subject to impending fracture. Nineteen fractures (13.9%) occurred in the former group and 14 (5%) in the latter; this difference was significant (P = 0.002). As for the Mirels’ score, 191 femurs were considered as not likely to be subject to impending fracture. Among these, 51 femurs had bone destruction that was not visible on X-ray images, but that was detectable with CT, MRI or PET. The remaining 140 femurs had Mirels’ scores ≤8. Six fractures occurred in these 191 patients (3.1% of patients). In contrast, 27 fractures occurred among 228 femurs that had Mirels’ scores ≥9 (11.8%). This difference was also significant (P = 0.001). Details of the Mirels’ scores and fracture rate are shown in Fig. 2.
Fig. 2.
Likelihood of fracture based on the scoring system of Mirels in 428 femoral metastases.
Pathological findings of post-irradiation fractures of femoral metastasis
Surgery was performed on 27 of 33 fractures; the remaining six fractures were treated conservatively because the patient’s general condition was poor or the patient refused surgery. The surgeries included endoprosthesis replacement in 12 femurs and osteosynthesis in 15. Pathological sampling of the fracture site was obtained in 24 patients (19 actual fractures and five painful virtual fractures) during surgery. Cancer cells persisted in 14 of 24 sampled femurs, while no remaining tumor cells were found in 10 (Table 3).
Among seven actual fractures that occurred within 3 months and from which pathological specimens were obtained, six (85.7%) showed remaining tumor cells microscopically. Among fractures that occurred after 3 months, only three of 12 femurs (25%) had tumor cells present; this difference was significant (P = 0.002). All painful virtual fractures showed viable tumor cells.
Patient background and post-radiation fracture
We did not find any significant differences between fracture and non-fracture groups with respect to background factors, including patient age, irradiation dose, or regular zoledronic acid administration (χ2 test) (Table 4).
Survival rate for all patients
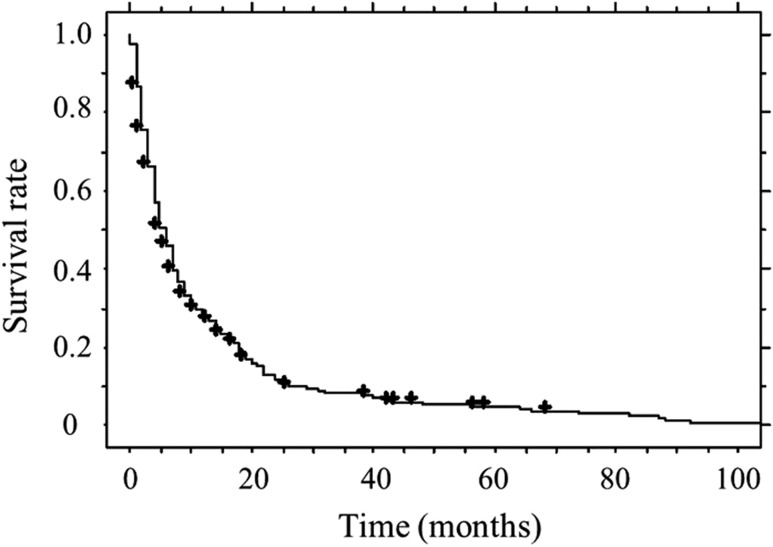
The median survival period for all patients was 5.5 months. The survival rates at 1, 2 and 3 years were 0.27, 0.12 and 0.08, respectively (Fig. 3).
Fig. 3.
Survival rates for all patients. Survival rates at 1, 2 and 3 years were 0.27, 0.12 and 0.08, respectively.
DISCUSSION
Radiotherapy is currently a mainstream treatment for metastatic bone tumors. Katagiri et al. [21] reported that among 808 patients with skeletal metastasis, 749 (93%) were treated non-surgically, and that 623 (77%) of these received radiotherapy. Even in cases in which osteosynthesis or spinal decompression procedures are required, surgery is sometimes impossible, and radiation therapy is indicated because of the patient’s poor general condition or patient refusal. Radiation therapy is a mainstream treatment in cases of femoral metastasis as well; however, physicians occasionally encounter PIFF.
The incidence of PIFF varies among previous reports, from 8.9% in the study by Keene et al. to 35% in that by Mirels [5–9, 11]. The incidence of PIFF in the present study was 7.7%, which is slightly lower than that of other studies. In the studies by Keene et al., the primary cancer was restricted to breast cancer. However, the primary cancers in the present study were not substantially different from those in the other reports (Table 5). This lower rate in our study might have resulted from our protocol instructing all patients with painful bone destruction to use crutches for 3 months after radiation therapy, which is the period required for bone regeneration [5]. Other reasons for our lower rate of PIFF could be that many of the patients in these three earlier studies were treated before the use of zoledronic acid became popular, or that the degree of bone destruction and the risk of fracture among our patients might have differed from those of other study populations.
Table 5.
Comparison with past reports regarding femoral fractures after radiation therapy
| Primary tumor site | Fracture rate (%) | Proximal fracture (%) | Time to fractures after RT (months) | Dose (Gy) | |
|---|---|---|---|---|---|
| Median (months) | |||||
| Keene et al. [7] | Breast | 8.9 | N/A | 2–18 N/A |
20–55 |
| Linden et al. [6, 8] | Breast, lung, prostate, other | 12.7 | 86 | 0–9 8.5 |
8–24 |
| Harada et al. [5] | Breast, lung, prostate, liver Thyroid, kidney, other |
9.5 | N/A | N/A N/A |
30–40 |
| Tatar et al. [9] | Breast, lung, prostate, other | 25.0 | N/A | 0–6.6 N/A |
7–30 |
| Current study | Breast, lung, prostate, liver Esophagus, colon, other |
7.7 | 91 | 0–21 4.4 |
20–39 |
N/A = Not applicable.
The Mirels’ score system [11] includes four factors for evaluating the risk of pathological fracture: the site of metastasis, pain, size of lesion, and X-ray findings. Each factor is scored from 1 to 3 points; combined scores of 9 points or more indicate impending fracture. According to this system, peritrochanteric lesions are scored as 3 points, while the lower limb is scored as 2 points, suggesting that peritrochanteric lesions have the highest risk of fracture. Keene et al. reported a fracture rate of 14% in the proximal femur and 0.7% in shaft lesions [7]. Linden et al. reported that 86% of fractures occurred in the proximal femur [6]. The current study also revealed that the proximal femur had a higher PIFF rate than the femoral shaft.
In this study, 5 of 33 femurs with post-irradiation fractures were affected by tumors with low sensitivity to radiation; all of these fractured early (three fractures within 3 months, two fractures within 4 months). These results indicate a tendency for early fracture following radiation therapy in metastases with low sensitivity to radiation therapy. In this study, there were no cases of renal cell carcinoma and only one case of thyroid cancer among PIFF patients. Femoral metastases with evident bone destruction from these two types of cancer are usually treated surgically, because they tend to present as impending fracture in a patient with good general condition and long life expectancy.
The interval from the completion of radiation therapy to PIFF has been briefly reported in the literature. Keene et al. reported that PIFF occurred at an average of 10 months, whereas Mirels et al. reported that all PIFFs occurred within 6 months. Linden et al. found that fracture occurred within a median of 8.5 weeks (2–36) and that 90% of fractures occurred within 6 months [6, 7, 11]. In the recent study by Tatar, all fractures occurred within 6.6 months [9]. In the present study, 39.3% of PIFFs occurred within 3 months, and 63.6% occurred within 6 months. PIFF in the current study developed later compared with the studies by Linden et al. and Mirels et al., but a similar tendency of early fracture development and a decrease over time was observed. This finding may be explained by the fact that femurs weakened by metastasis require several months to regain their strength after radiation therapy. Harada et al. [5] reported that radiological regeneration of bone was observed on plain X-ray images an average of 3 months after the completion of radiation therapy. Another reason for this finding may be the decreasing number of surviving patients over time. The number of surviving patients generally decreases over time among those with bone metastasis. Consequently, the number of fractures might decrease over time.
Only 13.9% of the impending fractures according to Harrington’s criteria eventually developed PIFF. Similarly, the fracture rate of femurs with Mirels’ score ≥9 was only 11.8%. Even among femurs with a score of 11, the fracture rate was only 20.8%. These results agree with those of Linden et al., who reported a fracture rate of 16.7% for femurs with a Mirels’ score ≥9 and 23.8% for those with a score of 11 [6]. Therefore, as suggested by Linden et al., deciding on the surgical indication based only on the Mirels’ score or Harrington’s criteria might lead to unnecessary surgery.
Persistent tumor cells were found more frequently in actual fractures that occurred within 3 months after radiation therapy (85.7%) than in those that occurred after 3 months (25.0%). Two reasons are presumed for this difference. First, >3 months may be necessary before the appearance of tumor cell necrosis after radiotherapy. Second, metastasis of tumors that are resistant to radiation therapy might result in early fractures because such metastases (including HCC, cholangiocellular carcinoma, and thyroid cancer) cause fractures early in this series. Approximately 70% of actual fractures occurring after 3 months did not show any remaining tumor cells. Therefore, we surmise that PIFF occurring after 3 months resulted primarily from bone fragility following radiation therapy. Hatano et al. [22] reported a case in which a femoral fracture occurred 10 years after radiation therapy. In that case, there were no remaining tumor cells and the fracture was caused by bone fragility resulting from radiation therapy.
Many studies have found that skeletal-related events are prevented or delayed by the administration of zoledronic acid [23, 24]. It has also been reported that performance status and quality of life improve in patients receiving radiotherapy combined with bisphosphonates [25]. An animal model study found a greater increase in bone density in those treated with combined bisphosphonates and radiotherapy than in those receiving radiation therapy alone [26]. However, in the current study, the fracture rate was not lower in the regular zoledronic acid administration group (9.5%) than among other patients (7.1%). Therefore, the usefulness of regular zoledronic acid administration in preventing PIFF could not be confirmed. This finding might have been caused by bias, because only 27.0% of the patients in this study were administered zoledronic acid regularly, and those patients with a higher risk of fracture might have been more likely to receive zoledronic acid treatment. Long-course radiotherapy was reportedly associated with better local control compared with short-course radiotherapy (8 Gy in a single fraction, 20 Gy in five fractions) in the treatment of spinal metastasis [12]. In contrast, Tatar et al. reported that procedures and fractionation did not affect fracture incidence [9]. Similarly, we did not find differences in the incidence of PIFF between long-course radiotherapy and short-course radiotherapy.
There were some limitations in this study. First, this was a retrospective study, and the number of fractures was small. Therefore, results concerning fracture timing and the fracture-preventing effect of bisphosphonates are not conclusive. A prospective study comprising a larger number of cases would be required for drawing more definitive conclusions. Second, because tissues were sampled from 24 of 33 fractures, research including more samples is necessary to validate the relationship between tumor remnants and fracture timing. Third, 28 femurs that initially underwent prophylactic surgery were not included in this study. Generally speaking, these lesions were deemed to be clearly subject to impending fracture and were considered appropriate for surgery. Therefore, the PIFF incidence might have been lowered by the exclusion of these 28 femurs.
The current study demonstrated that PIFF occurred most often within the first 3 months after radiotherapy and occurred more frequently in the proximal femur than in the shaft. Therefore, it is recommended that patients undergoing radiotherapy for proximal femoral metastasis be informed of the risk of fracture and that a few months of partial weight-bearing with a crutch be prescribed. The occurrence of actual fracture within 3 months after radiotherapy or painful virtual fracture after radiotherapy may indicate a high likelihood of remaining viable tumor cells. Therefore, when performing surgery under these situations, orthopedic surgeons must take care not to contaminate the surgical field with tumor cells, especially in patients undergoing excision followed by prosthetic replacement.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Anderson PR, Coia LR. Fractionation and outcomes with palliative radiation therapy. Semin Radiat Oncol 2000;10:191–9. [DOI] [PubMed] [Google Scholar]
- 2. Meeuse JJ, van der Linden YM, van Tienhoven G, et al. . Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: results from the Dutch Bone Metastasis Study. Cancer 2010;116:2716–25. [DOI] [PubMed] [Google Scholar]
- 3. Mizumoto M, Harada H, Asakura H, et al. . Prognostic factors and a scoring system for survival after radiotherapy for metastases to the spinal column: a review of 544 patients at Shizuoka Cancer Center Hospital. Cancer 2008;113:2816–22. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura N, Shikama N, Wada H, et al. . Patterns of practice in palliative radiotherapy for painful bone metastases: a survey in Japan. Int J Radiat Oncol Biol Phys 2012;83:e117–20. [DOI] [PubMed] [Google Scholar]
- 5. Harada H, Katagiri H, Kamata M, et al. . Radiological response and clinical outcome in patients with femoral bone metastases after radiotherapy. J Radiat Res 2010;51:131–6. [DOI] [PubMed] [Google Scholar]
- 6. Van der Linden YM, Dijkstra PD, Kroon HM, et al. . Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br 2004;86:566–73. [PubMed] [Google Scholar]
- 7. Keene JS, Sellinger DS, Mcbeath AA, et al. . Metastatic breast cancer in the femur. A search for the lesion at risk of fracture. Clin Orthop Relat Res 1986;203:282–8. [PubMed] [Google Scholar]
- 8. Van der Linden YM, Kroon HM, Dijkstra SP, et al. . Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: results from a randomized trial. Radiother Oncol 2003;69:21–31. [DOI] [PubMed] [Google Scholar]
- 9. Tatar Z, Soubrier M, Dillies AF, et al. . Assessment of the risk factors for impending fractures following radiotherapy for long bone metastases using CT scan-based virtual simulation: a retrospective study. Radiat Oncol 2014;9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harrington KD. Orthopaedic Management of Metastatic Bone Disease. St Louis: The C. V. Mosby Company, 1988, 283–308. [Google Scholar]
- 11. Mirels H. Metastatic disease in long bones: a proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res 2003;415:S4–13. [DOI] [PubMed] [Google Scholar]
- 12. Rades D, Lange M, Veninga T, et al. . Final results of a prospective study comparing the local control of short-course and long-course radiotherapy for metastatic spinal cord compression. Int J Radiat Oncol Biol Phys 2011;79:524–30. [DOI] [PubMed] [Google Scholar]
- 13. Patrick SS, Steven L, David CP. Cancer of the thyroid In: Steven AL, Theodore LP(eds). Textbook of Radiation Oncology. 2nd edn Philadelphia: Saunders Elsevier, 2004, 757–78. [Google Scholar]
- 14. He J, Zeng ZC, Tang ZY, et al. . Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer 2009;115:2710–20. [DOI] [PubMed] [Google Scholar]
- 15. Raquel TW, Robin S. Cancer of the liver, bile duct and gallbladder In: Steven AL, Theodore LP (eds). Textbook of Radiation Oncology. 2nd edn Philadelphia: Saunders Elsevier, 2004,857–84. [Google Scholar]
- 16. Duncan M. Kidney, bladder, prostate, testis, urethra, penis In: Bomford CK, Kunkler IH, Hancock BW (eds). Walter and Miller’s Textbook of Radiotherapy. Radiation Physics, Therapy and Oncology. 6th edn Edinburgh: Churchill Livingstone Elsevier, 2003, 487–507. [Google Scholar]
- 17. Jung IH, Yoon SM, Kwak J, et al. . High-dose radiotherapy is associated with better local control of bone metastasis from hepatocellular carcinoma. Oncotarget 2017;8:15182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Varadarajan VV, Pace EK, Patel V, et al. . Follicular thyroid carcinoma metastasis to the facial skeleton: a systematic review. BMC Cancer 2017;17:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Capanna R, Campanacci DA. Indications for the surgical treatment of long bone metastases In: Jasmin C, Coleman RE, Coia LR, et al. (eds). Textbook of Bone Metastases. 1st edn West Sussex, England: Wiley, 2005, 136–46. [Google Scholar]
- 20. Mizumoto M, Harada H, Asakura H, et al. . Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys 2011;79:208–13. [DOI] [PubMed] [Google Scholar]
- 21. Katagiri H, Okada R, Takagi T, et al. . New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014;3:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatano H, Morita T, Kobayashi H, et al. . Pathological fracture of the femur ten years after successful radiation therapy for metastatic breast cancer. Breast Cancer 2004;11:313–7. [DOI] [PubMed] [Google Scholar]
- 23. Kohno N, Aogi K, Minami H, et al. . Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 2005;23:3314–21. [DOI] [PubMed] [Google Scholar]
- 24. Lipton A, Colombo-Berra A, Bukowski RM, et al. . Skeletal complication in patients with bone metastases from renal cell carcinoma and therapeutic benefits of zoledronic acid. Clin Cancer Res 2004;10:6397S–403S. [DOI] [PubMed] [Google Scholar]
- 25. Kouloulias V, Matsopoulos G, Kouvaris J, et al. . Radiotherapy in conjunction with intravenous infusion of 180 mg of disodium pamidronate in management of osteolytic metastases from breast cancer: clinical evaluation, biochemical markers, quality of life, and monitoring of recalcification using assessments of gray-level histogram in plain radiographs. Int J Radiat Oncol Biol Phys 2003;57:143–57. [DOI] [PubMed] [Google Scholar]
- 26. Vassiliou V, Kardamakis D. The management of metastatic bone disease with the combination of bisphosphonates and radiotherapy: from theory to clinical practice. Anticancer Agents Med Chem 2009;9:326–35. [DOI] [PubMed] [Google Scholar]