Abstract
Background
Oxytocin may be a possible treatment for multiple neuropsychiatric disorders, including cocaine addiction. Little is known about the site-specific effects of oxytocin on various drug addiction-related brain regions. Furthermore, sexually dimorphic effects of oxytocin on neural function in the addiction circuit have not been established. Here, we studied Fos expression following cocaine-cued reinstatement in both male and female rats.
Methods
Male and female rats underwent self-administration, extinction, and reinstatement tests. On test days, rats were given oxytocin or vehicle, and lever pressing was measured in response to conditioned cocaine cues. Rats were perfused and Fos staining measured in the central amygdala, medial prefrontal cortex, nucleus accumbens core, and subthalamic nucleus. Fos/oxytocin double labeling occurred in the paraventricular nucleus of the hypothalamus.
Results
Rats reinstated to cocaine cues relative to extinction responding and oxytocin reduced cocaine seeking. Oxytocin combined with contingent cue presentations increased Fos+ oxytocin cell bodies within the paraventricular nucleus of the hypothalamus relative to vehicle. Fos expression robustly increased in the central amygdala following oxytocin administration. Oxytocin reversed cue-induced Fos expression in the medial prefrontal cortex, nucleus accumbens core, and subthalamic nucleus. Central oxytocin infusion also attenuated reinstated cocaine seeking.
Conclusions
Oxytocin decreased reinstated cocaine seeking, increased Fos activation in the paraventricular nucleus of the hypothalamus and central amygdala, but normalized cue-induced Fos activation in the medial prefrontal cortex, nucleus accumbens core, and subthalamic nucleus, thereby demonstrating regionally specific activation patterns. No sex differences were seen for the effects of oxytocin on cocaine seeking and Fos activation, indicating that oxytocin acts on similar central neural circuits critical to reinstated cocaine seeking in both males and females.
Keywords: neuroactive peptides, substance abuse, oxytocin, addiction
Significance Statement
Oxytocin is a neuroactive peptide that is being considered as a therapeutic for cocaine addiction. In animal models, oxytocin consistently decreases relapse to cues that are associated with the physiological effects of the drug. The neural mechanisms driving this ability remain unclear and are the focus of this paper. Here, we used the immediate early gene Fos as a marker of neuronal activation during responding for cocaine cues to identify neural activity in brain areas associated with drug addiction.
Oxytocin Reduces Cocaine-Cued Fos Activation in a Regionally Specific Manner
Oxytocin is a well-characterized neuroendocrine hormone produced in the mammalian hypothalamus that is recognized as an important modulator of numerous behavioral functions such as emotional regulation, social/maternal interactions, and sexual behavior (Gimpl and Fahrenholz, 2001). Oxytocin has sex-specific effects in male and female reproductive behaviors, including modulation of uterine contractions during labor and postpartum lactation in females (Tahara et al., 2002) and inducing penile erection in males (Melis et al., 2007). Sexual dimorphisms also exist in the central oxytocin system. For example, female mice display an increased number of oxytocin cells, axons, and cellular levels in the hypothalamus relative to male mice (Haussler, Jirikowski and Caldwell, 1990). However, this difference is absent in other rodent species (Wang et al., 2013) and is also absent in humans (Ishunina and Swaab, 1999). Male and female rats also differ in their distribution of oxytocin receptors within the reward circuitry (Dumais et al., 2013), with females displaying lower receptor expression in regions such as the nucleus accumbens, caudate putamen, and medial amygdala compared with males.
Cocaine addiction continues to be a societal and economic problem that remains without an effective form of pharmacotherapy. Oxytocin has gained attention as a viable therapeutic target for treatment of drug addiction (Sarnyai and Kovacs, 2014; Baracz and Cornish, 2016), as increasing evidence points towards the ability of oxytocin to attenuate drug seeking and craving. For example, oxytocin blocked a preference for a methamphetamine-paired environment (Carson et al., 2010a), attenuated methamphetamine seeking (Qi et al., 2009; Carson et al., 2010a; Cox et al., 2013, 2016), reduced ethanol (King et al., 2017) and heroin (Kovacs et al., 1985) self-administration, and decreased morphine tolerance and withdrawal (Kovacs et al., 1985; Sarnyai and Kovacs, 1994). Similarly, the oxytocin analogue carbetocin reversed stress- (Zanos et al., 2014) and morphine-induced (Georgiou et al., 2014) reinstatement of morphine conditioned place preference (CPP). In regards to cocaine, systemic oxytocin reduced cocaine-induced sniffing behavior (Sarnyai et al., 1991), and this behavior was attenuated by an intracerebral infusion of an oxytocin receptor antagonist (Sarnyai and Kovacs, 1994). Recent cocaine self-administration studies from our laboratory have shown that systemic oxytocin decreased active lever presses, cocaine intake, and conditioned cue-induced cocaine seeking following extinction in both males (Zhou et al., 2015) and females (Leong et al., 2016).
Sex differences have been well characterized in the formation and maintenance of cocaine addiction. Female rats show greater motivation to seek cocaine relative to males across different stages of addiction (for review, see Becker, McClellan, and Reed, 2017) and typically reinstate to a greater extent following a cocaine prime or a pharmacological stressor (Kippin et al., 2005; Anker and Carroll, 2011; Feltenstein, Henderson and See, 2011; Buffalari et al., 2012). In contrast, females typically reinstate less than or equally to males in response to drug-conditioned cues (Fuchs et al., 2005; Feltenstein et al., 2011; Cox et al., 2017). Both peripheral and central oxytocin administration attenuated cocaine seeking in males (Morales-Rivera et al., 2014), and we have shown that peripheral oxytocin also reduced cocaine seeking in females (Leong et al., 2016). Few studies have directly compared sex differences in neuronal function in response to cocaine-conditioned cues. One study demonstrated that the relationship between neuronal activation (as determined by Fos expression) and cocaine seeking might be similar in male and female rats in brain areas related to the relapse circuit (Zhou et al., 2014). Previously, we demonstrated that Fos expression was increased in the nucleus accumbens core (NAc) following methamphetamine-cued reinstatement in males, and oxytocin returned this elevation to control levels (Cox et al., 2017). The present study investigated the effects of oxytocin on cue-induced reinstatement of cocaine seeking along with corresponding neuronal activation (e.g., regional Fos expression) in both male and female rats. Specifically, we looked at the number of Fos+ oxytocin cells in the paraventricular nucleus (PVN) and examined 4 forebrain areas relevant to cued reinstatement: central amydala (CeA), medial prefrontal cortex (mPFC), nucleus accumbens core (NAc), and subthalamic nucleus (STN).
Methods and Procedures
Subjects
Age-matched adult male (weighing 275–300 g) and female (weighing 205–225 g) Sprague Dawley rats (Harlan; n=92) were single-housed in a temperature- and humidity-controlled vivarium on a reversed 12-hour-light:-dark cycle (lights off at 6:00 am). All surgical and experimental procedures were conducted during the dark cycle. Rats were kept on a daily “stable intake” diet (15–30 g) of standard rat chow (Harlan) for the duration of the study and received water ad libitum. All surgical and experimental procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011) and approved by the IACUC of the Medical University of South Carolina.
Surgery
Rats were anesthetized with ketamine (66 mg/kg, i.p., Vedco Inc) mixed with xylazine (1.3 mg/kg, i.p., Lloyd Laboratories), and an additional injection of Equithesin (0.5 mL/kg: sodium pentobarbital 9.72 mg/kg, chloral hydrate 42.5 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10% ethanol solution) and cefazolin (100 mg/kg; SC; West-ward Pharmaceuticals). Ketorolac (2.0 mg/kg, i.p., Sigma) was given before surgery as an analgesic. One end of a silastic catheter was implanted into the external right jugular. The other end ran subcutaneously, exited from a small incision on the back, and attached to an infusion cannula (PlasticsOne Inc.). For central drug infusion, rats were mounted onto a stereotaxic apparatus following catheter implantation. Cannulae were bilaterally aimed at the lateral ventricles (-1.0 AP; ±2.2 ML; -1.9 DV; 10º angle relative to the skull surface and bregma), secured using jeweler’s screws and dental acrylic, and stainless-steel obturators were then inserted. All surgical procedures were conducted using aseptic techniques. Cephazolin (10 mg) was given postsurgery (0.1 mL i.v.) and during recovery along with 0.05 mL of taurolidine-citrate catheter locking solution (TCS). Rats were given at least 5 days to recover from surgery.
Cocaine Self-Administration, Extinction, and Reinstatement
All behavioral training occurred in self-administration chambers (30×20×20 cm, Med Associates) containing 2 retractable levers on one side, 2 stimulus lights (on 2 opposite walls), an ambient house light, and a speaker. Self-administration chambers were placed inside sound-attenuating cubicles with a fan to provide ambient noise. All chambers contained tubing that ran through a spring leash attached to a swivel and a balanced metal arm. A 10-mL syringe was placed on a pump outside each chamber to supply infusions of cocaine. Cocaine hydrochloride (provided by the National Institute on Drug Abuse, Research Triangle Park) was dissolved in 0.9% sterile saline and administered at 0.20 mg cocaine per 50-μL bolus for males and 0.15 mg per 50-μL bolus for females, based on the proportional body weight difference between male and female rats. Collection of data was controlled through a software program (MED-PC, Med Associates). Cocaine self-administration occurred in daily 2-hour sessions along a fixed ratio 1 schedule of reinforcement. During self-administration, a response on the active lever resulted in a 2-second infusion and 5-second presentation of a light and tone stimulus complex, followed by a 20-second time out. Responses occurring during the time out and on the inactive lever were recorded without scheduled consequences. Rats were trained to self-administer cocaine until they reached a criterion of 10 consecutive days with >10 infusions. All rats reached criteria between 10 and 14 days. Before each session, catheters were flushed with 0.1 mL of saline. To verify catheter patency, rats received a 0.10- to 0.12-mL infusion of methohexital sodium (Eli Lilly), a short-acting barbiturate that produces a rapid loss of muscle tone when administered i.v., as needed. After each self-administration session, rats’ catheters were flushed with 0.05 mL TCS.
All rats underwent extinction following completion of cocaine self-administration. Daily extinction sessions lasted 2 hours and continued for 7 to 12 days, during which responding on either lever had no scheduled consequences. Extinction criterion was ≤25 active lever presses for 2 consecutive days. Upon meeting the criterion, rats were tested on conditioned cued reinstatement tests, in which responding on the active lever now resulted in the presentation of the light and tone stimulus complex but no cocaine infusion. Test sessions were 90 minutes and each rat tested twice. In experiment 1, all rats received oxytocin (1 mg/kg, i.p.) and saline in a counterbalanced order with 3 to 5 extinction sessions occurring between tests. In experiment 2, during extinction, all rats received an angiotensin test first to confirm cannula function in the ventricles (Buffalari et al., 2012) and one reinstatement test. During reinstatement testing, rats received either oxytocin (3 µg/0.5 µL/side) or vehicle 5 minutes prior to test. Thus, over both experiments, the number of test sessions was limited to 2 tests. We have used multiple cued reinstatement tests in the past and have not encountered order effects (Feltenstein and See, 2006; Fuchs, Feltenstein and See, 2006; Reichel and See, 2012; Cox et al., 2013; Leong et al., 2016).
Immunohistochemistry
Subjects in experiment 1 were sacrificed and perfused immediately after cued reinstatement testing and tissue was collected. Briefly, rats were deeply anesthetized with Equithesin and then transcardially perfused with 150 to 200 mL cold 0.9% saline followed by 400 to 500 mL of 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde for 24 hours, submerged in 20% sucrose for 48 hours, and then sectioned into 40-µm tissue sections. For Fos expression, tissue sections were incubated in a rabbit anti-Fos primary antibody (Calbiochem; 1:1000) overnight, followed by 2-hour incubation in donkey anti-rabbit secondary antibody (Jackson ImmunoResearch; 1:500) amplified with an avidin biotin complex method (Thermo Scientific). The sections were then visualized with 3,3’ diaminobenzidine (Sigma) + nickel ammonium sulfate to produce a blue-black nuclear reaction product. Brain sections containing the PVN were separated, and oxytocin was visualized with a mouse anti-oxytocin antibody (Millipore; 1:1000), donkey anti-mouse secondary (Jackson ImmunoResearch; 1:500), avidin biotin complex, and 3,3’ diaminobenzidine to yield a brown somatic stain at oxytocin cell bodies.
Quantification of Fos and Oxytocin-Expressing Cells
Blue-black nuclear immunoprecipitate from Fos positive cells and brown somatic stain at oxytocin cell bodies in the PVN were quantified using a brain atlas for comparison (Paxinos and Watson, 2007). The atlas was also used to identify Fos labeling in the other targeted nuclei. These regions were photographed at 10x magnification using a Leica microscope and VideoToolbox software. All images were quantified using ImageJ software (NIH). Fos-positive (Fos+) cells that fell within each region of interest were counted and averaged across sections for each rat. On average, 3 bilateral sequential sections for each region were used for analysis. Anterior-posterior coordinates for each analyzed region are as follows: mPFC (prelimbic)= +2.80 to +2.50; NAc = +1.30 to +1.00; CeA: -2.00 to -2.40; PVN: -2.5 to -2.75; and STN: -3.20 to -3.75. For the PVN only, Fos+ oxytocin cell bodies were counted and compared as a percentage of total oxytocin cell bodies in that region.
Data Analysis
Two-way mixed ANOVA was used to evaluate lever presses, cocaine intake, and extinction responding. Cocaine intake was calculated with the following formula ([number of infusions*cocaine dose]/body weight in kg). The between-subject variable was sex (males and females) and the repeated measure was day (self-administration days 1–10 and extinction days 1–8). Two-way ANOVAs were also used to analyze lever responses during cue-induced reinstatement tests and regional Fos activation. The variables were sex (males and females) and test condition (ext, cue-saline, and cue-oxy). All pairwise comparisons were conducted using Holm Sidak multiple comparisons test. For graphical purposes, when sex differences existed, data are presented separately for males and females. In the absence of sex differences, data were collapsed across the variable. In this case, the means and SEM for both sexes are presented in Tables 1 and 2. All data are shown as the mean ± SEM, and α was set at P < .05.
Table 1.
Active and Inactive Lever Presses during Reinstatement Testing in Male and Female Rats
| CUE+VEH (mean ± SEM) |
CUE+OXY (mean ± SEM) |
|
|---|---|---|
| Exp 1a: Males active | 57.61 ± 8.65 | 28.11 ± 5.15 |
| inactive | 7.65 ± 1.42 | 3.84 ± 1.28 |
| Exp 1a: Females active | 43.84 ± 7.63 | 17.16 ± 4.66 |
| inactive | 3.70 ± 1.21 | 2.26 ± 0.67 |
| Exp 2: Males active | 55.22 ± 2.78 | 33.42 ± 3.80 |
| inactive | 6.44 ± 1.70 | 4.43 ± 1.45 |
| Exp 2: Females active | 58.00 ± 6.31 | 37.22 ± 3.80 |
| inactive | 1.44 ± 0.71 | 1.56 ± 9.96 |
Table 2.
Average Fos-Positive Cells during Reinstatement Testing in Male and Female Rats
| EXT (mean ± SEM) |
CUE+VEH (mean ± SEM) |
CUE+OXY (mean ± SEM) |
|
|---|---|---|---|
| PVN (Fos+ Oxy): males | 13.73 ± 4.97 | 9.49 ± 1.62 | 45.22 ± 6.90 |
| PVN (Fos+ Oxy): females | 24.32 ± 2.47 | 22.38 ± 4.17 | 45.25 ± 9.19 |
| CeA: males | 19.95 ± 0.77 | 33.43 ± 7.06 | 90.25 ± 17.84 |
| CeA: females | 16.88 ± 2.60 | 22.69 ± 5.10 | 80.75 ± 17.5 |
| mPFC: males | 153.60 ± 8.61 | 191.30 ± 14.42 | 151.90 ± 15.07 |
| mPFC: females | 128.00 ± 11.27 | 179.50 ± 15.85 | 125.20 ± 8.19 |
| NAc: males | 54.96 ± 8.86 | 65.66 ± 6.10 | 50.10 ± 10.24 |
| NAc: females | 60.09 ± 7.03 | 83.22 ± 10.23 | 59.55 ± 6.15 |
| STN: males | 1.15 ± 0.66 | 15.50 ± 1.89 | 6.69 ± 1.94 |
| STN: females | 1.75 ± 0.33 | 14.99 ± 2.61 | 4.73 ± 1.34 |
Experiment 1: Effect of Systemic Oxytocin on Fos Expression during Cue-Induced Reinstatement in Male and Female Rats
In this experiment, males and females (n=48) underwent catheter implantation surgery followed by self-administration, extinction, and reinstatement as described above. Rats underwent 2 reinstatement tests with a minimum of 2 extinction days between each test until extinction criteria were met. Thirty minutes before reinstatement testing, rats received oxytocin (1 mg/kg; i.p.) or vehicle (Cox et al., 2013; Zhou et al., 2015; Leong et al., 2016). The 3 different test conditions consisted of: (1) the extinction condition (Ext) received a saline injection before a standard extinction session, (2) the Cue+Veh group received an injection of saline prior to a cued reinstatement test, and (3) the Cue+Oxy received oxytocin before the cued reinstatement test. During the reinstatement test, rats were placed into the test chamber and responding on the active lever resulted only in the presentation of the light and tone stimulus complex, but no infusion of cocaine. Cued reinstatement tests lasted for 90 minutes. Immediately following the second reinstatement test, rats were sacrificed and tissue was collected as described above.
Experiment 2: Effect of Central Oxytocin on Cue-Induced Reinstatement in Male and Female Rats
Males and females (n= 59) underwent catheter surgery and ICV cannulation followed by self-administration, extinction, and reinstatement. Angiotensin drinking tests were carried out following extinction trials on days 3 and 4 of extinction training to verify accurate cannula placement (Buffalari et al., 2012). Injectors were lowered into the cannula, and angiotensin (10 ng/mL) was administered bilaterally over a period of 60 seconds. Rats then had immediate access to drinking water for 10 minutes. Males that drank <6 mL and females that drank <5 mL of water were excluded from the study. Based on these criteria, 3 rats were excluded from the study. Rats that passed the drinking test but had a single blocked cannula (n=3) were tested on vehicle. Five minutes before reinstatement testing, rats received ICV infusions of oxytocin (3 µg/0.5 µL/side) or vehicle (Morales-Rivera, 2014; Cox et al., 2017). Similar to experiment 1, the 3 different test conditions consisted of: (1) an extinction test with a vehicle infusion before the session, (2) the Cue+Veh test with a central infusion of vehicle prior to cued reinstatement test, and (3) the Cue+Oxy condition using central infusion of oxytocin prior to cued reinstatement.
Results
Experiment 1: Systemic Oxytocin Altered Fos Expression during Cue-Induced Reinstatement in Male and Female Rats
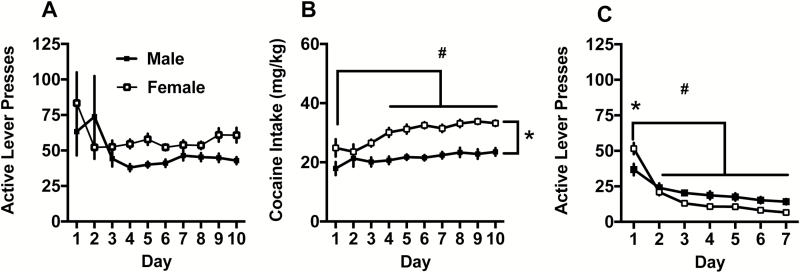
Active lever presses during self-administration are depicted in Figure 1A. The range in the number of self-administration sessions to reach criteria was 10 to 14 for both males and females. Males and females responded equally across the 10 days of cocaine self-administration. The 2-way ANOVA did not detect any main effects or interactions on active lever presses. Both sexes had clear lever discrimination throughout the self- administration period (supplementary Figure 1). Cocaine intake in mg/kg is depicted in Figure 1B. Females took more cocaine than males, as indicated by a significant main effect of sex [F(1,43)= 39.21, P<.0001]. For both sexes, cocaine intake increased over time, as seen by a main effect of day [F(9,387)= 6.67, P<.05], with days 4 to 10 being greater than day 1. The sex x day interaction was not significant [F(9,387)= 1.78, P= .07]. Lever responding during extinction is depicted in Figure 1C. There was a significant sex x day interaction during extinction [F(6,264)= 7.77, P<.0001], and females showed more lever presses on day 1 of extinction (Holm Sidak, P<.05). There was also a main effect of day [F(6,264)= 62.47, P<.0001], with responding on days 2 to 7 lower than extinction day one in both males and females (Holm Sidak, P<.05). The main effect of sex was not significant [F(1,44)= 2.51, P= .12].
Figure 1.
(A) Active lever presses during self-administration. (B) Cocaine intake (mg/kg) during self-administration. Females took more cocaine than males (*P<.05), and cocaine intake increased relative to the day 1 (#P<.05). (C) Females responded more on extinction day 1 relative to males (*P<.05), and active lever presses significantly decreased compared with day 1 on all ensuing days (#P<.05).
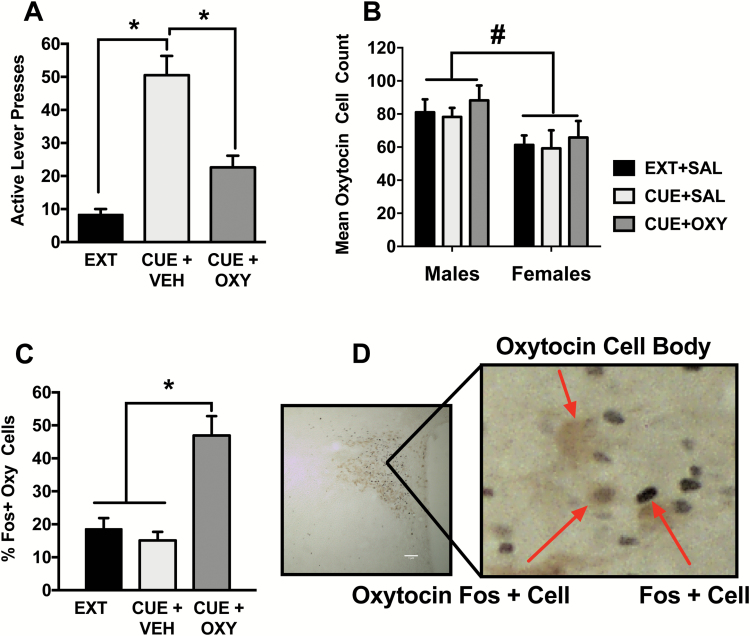
Lever responding during the cued reinstatement test is depicted in Figure 2A. The 2-way ANOVA detected a main effect of test condition [F(2,84)=17.22, P<.0001] on active lever presses. The Cue+Sal condition showed robust reinstatement relative to Ext and Cue+Oxy conditions (Holm Sidak, P<.05). Responding between the Ext and Cue+Oxy conditions did not differ on cued reinstatement (Holm Sidak, P> .05). The main effects of sex [F(1,84)=2.91, P= .09) and sex x test condition [F(2,84)= 0.09, P= .91] interaction were not significant. On the inactive lever, there was a main effect of sex with males responding more in general [F(1,83)= 6.11, P<.05] and a main effect of test condition [F(2,83)= 3.53, P<.05]. Posthoc comparisons on the test condition main effect were not significant. also, there was no sex x test condition interaction [F(2,83)= 0.58, P> .05]. Table 1 presents the means and SEM for both males and females on the active and inactive levers for comparison purposes.
Figure 2.
(A) Active lever presses after cued reinstatement. Rats robustly responded on the cue-associated lever in the Cue+Veh condition relative to extinction (Ext) and Cue+Oxy (*P<.05, significant difference between test conditions). (B) Average counts of oxytocin cell bodies in the paraventricular nucleus (PVN) in males and females across all test conditions. Males had more oxytocin positive cell bodies relative to females (#P<.05, significant difference between sexes). (C) Oxytocin administration increased Fos+ oxytocin cells in the PVN in rats tested with the Cue+Oxy condition relative to Ext and Cue+Veh (*P<.05, significant difference between test conditions). (D) Depiction of the quantification of Fos+ oxytocin cell bodies in the PVN.
Figure 2B shows that males had a greater number of oxytocin cell bodies within the PVN relative to females, indicated by a main effect of sex [F(1,33)= 8.6, P<.01]. This main effect of sex in PVN oxytocin cell body expression was the only sex difference that emerged in any of the analyses. There was no main effect of test condition [F(2,33)= 0.52, P= .60] or sex x test condition interaction [F(2,33)= 0.02, P= .98]. In the PVN, oxytocin treatment increased the percentage of Fos-+ oxytocin cell bodies. Specifically, there was a main effect of test condition [F(2,35)= 17.83, P<.0001], with Cue+Oxy showing the greater percentage of Fos+ oxytocin neurons than extinction and Cue+Veh tests (Holm Sidak, P<.05) (Figure 2C). The main effect of sex [F(1,35)= 3.15, P= .08] and the sex x test condition interaction were not significant [F(2,35)= 0.82, P= .45].
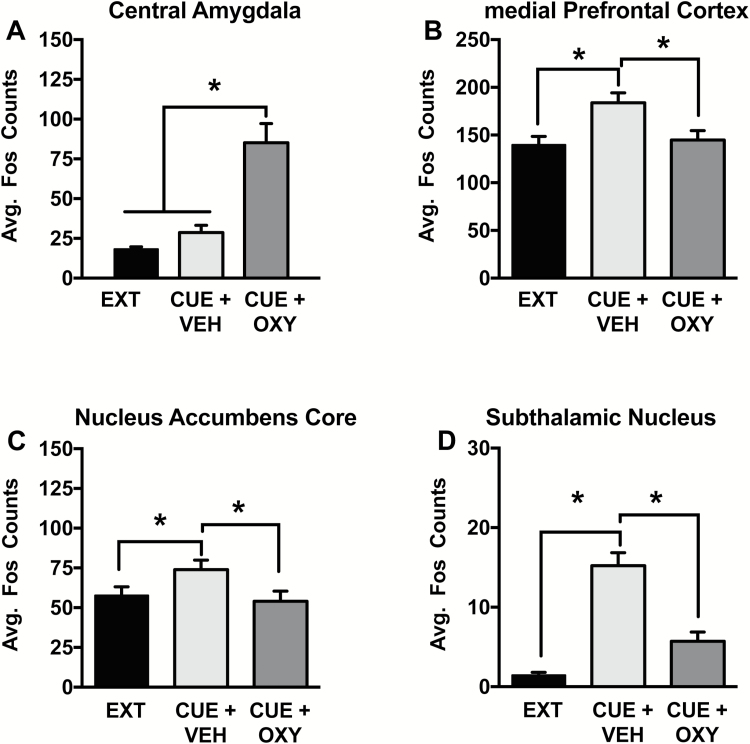
In the CeA (Figure 3A), oxytocin before the cue test increased Fos+ cell expression, indicated by a main effect of test condition [F(2,36)= 21.35, P<.0001]. More specifically, Cue+Oxy resulted in a higher number of Fos+cells than the extinction and Cue+Veh conditions (Holm Sidak, P<.05). The main effect of sex [F(1,36)= 0.76, P= .39] and the sex x test condition interaction were not significant [F(2,36)= 0.06, P= .94].
Figure 3.
Effect of peripheral oxytocin administration on region specific Fos expression during cued reinstatement. (A) In the central amydala (CeA), Cue+Oxy increased Fos expression relative to extinction (Ext) and Cue+Veh during cued reinstatement. (B) In the prefrontal cortex (PFC), (C) nucleus accumbens core (NAc), and (D) subthalamic nucleus (STN), Cue+Veh increased Fos expression relative to Ext and Cue+Oxy. Significant difference between test conditions (*P<.05).
Very similar patterns of Fos positive cells were found in the mPFC, NAc, and STN. In all 3 areas, increased Fos+ expression occurred during cued reinstatement in the saline condition, an effect that was normalized by oxytocin administration. Main effects of test condition were seen for the PFC [Figure 3B; F(2,31)= 8.43, P<.001], NAc [Figure 3C, F(2,38)= 3.3, P<.05], and the STN [Figure 3D, F(2,25)= 11.77, P<.0005]. More specifically, the Cue+Veh condition had a higher number of Fos+cells than the Ext and the Cue+Oxy conditions (Holm Sidak, P< .05). The main effects of sex [PFC: F(1,36)= 3.91, P= .06; NAc: F(1,38)= 2.35, P= .13; STN: F(1,25)= 0.13, P= .72] and the sex x test condition interactions [PFC: F(2,31)= 0.21, P= .81; NAc: F(2,38)= 0.31, P= .74; STN: F(2,25)= 0.79, P= .47] were not significant.
Experiment 2: Central Oxytocin (ICV) Administration Attenuated Cue-Induced Cocaine-Seeking
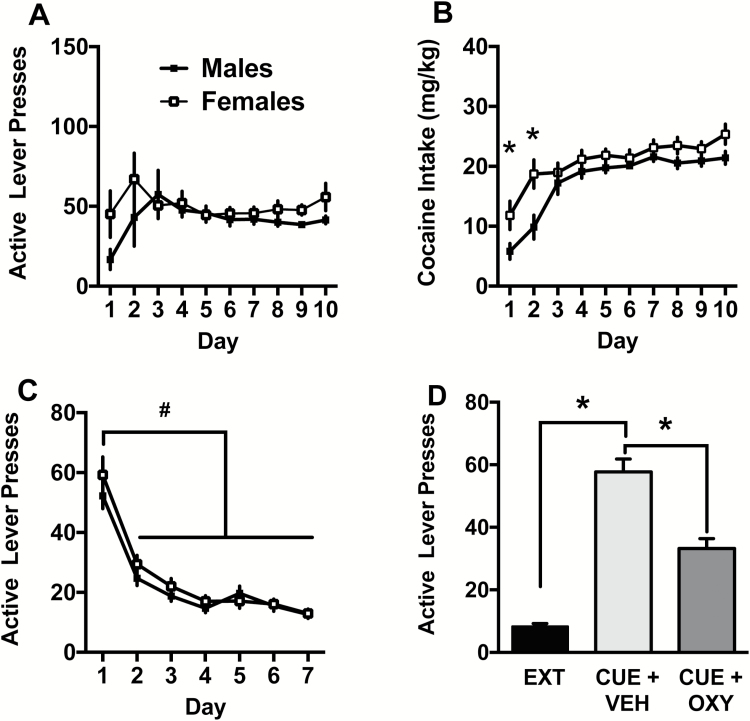
Lever presses during self-administration are depicted in Figure 4A. All males and females reached criteria in 10 days of self-administration. There were no differences in active lever responding between males and females over the 10 days at criteria. Both sexes had clear lever discrimination throughout the self-administration period (supplementary Figure 1). Cocaine intake (mg/kg) is depicted in Figure 4B. The 2-way ANOVA detected an interaction between sex and day [F(9,522)= 2.02, P<.05]. Specifically, females took more cocaine than males on the first 2 days of self-administration. The main effects of day [F(9,522)= 27.88, P<.001] and sex [F(1,58)= 7.19, P<.001] were also significant. Lever responding during extinction is depicted in Figure 4C. Males received between 7 and 11 days of extinction training and females received between 7 and 12 days of extinction training. There was a main effect of extinction day [F(6,324)= 89.38, P<.0001], with responding on days 2 to 7 lower than extinction day 1 in both males and females (Holm Sidak, P<.05). The main effect of sex [F(1,54)= 0.76, P= .39] and the sex x extinction day interaction [F(6,324)= 1.02, P= .42] were not significant.
Figure 4.
(A) Active lever presses during self-administration. (B) Cocaine intake (mg/kg) during self-administration. Females took more cocaine than males on days 1 and 2 (*P<.05). (C) During extinction, active lever presses significantly decreased compared with day 1 on all ensuing days (#P<.05). (D) Active lever presses during cued reinstatement. Rats robustly responded on the cue-associated lever in the Cue+Veh condition relative to extinction (Ext) and Cue+Oxy. Significant difference between test conditions (*P<.05).
Lever responding during the cued reinstatement test is depicted in Figure 4D. The 2-way ANOVA detected a main effect of test condition [F(2,43)= 65.09, P<.0001] on active lever presses. The Cue+Sal condition showed robust reinstatement relative to Ext and Cue+Oxy conditions (Holm Sidak, P<.05), and the Cue+Oxy condition showed greater reinstatement values than Ext condition (Holm Sidak, P<.05). The main effect of sex [F(1,43)= 3.96, P= .053] approached significance, but the sex x test condition [F(2,43)= 1.56, P= .22] interaction was not significant. On the inactive lever, there was a main effect of sex with males responding more in general [F(1,43)= 12.67, P<.009], but the main effect of test condition [F(2,43)= 0.06, P> .05] and sex x test condition interaction [F(2,43)= 0.73, P> .05] were not significant. Table 1 presents the means and SEM for both males and females on the active and inactive levers for comparison purposes.
Discussion
Here, we measured regional Fos expression following a cued reinstatement test to determine whether systemic oxytocin would impact neural activation in response to cocaine-conditioned cues. Males had a greater number of oxytocin immunoreactive neurons relative to females in the cell body region (i.e., the PVN), and peripheral oxytocin increased the number of Fos+ oxytocin neurons similarly in both sexes in response to cocaine cues. Likewise, oxytocin increased Fos+ neurons in the CeA relative to vehicle. Fos expression was increased relative to extinction levels in response to drug-conditioned cues and oxytocin normalized Fos expression in addiction-related regions of the NAc, the mPFC, and the STN. These data indicate regionally specific patterns of oxytocin-induced Fos expression. Importantly, changes in Fos positive immunoreactivity occurred following peripheral oxytocin administration, adding to a growing body of literature demonstrating that systemic oxytocin exerts various effects on central function. Furthermore, the overall lack of sex differences in oxytocin’s ability to decrease regional Fos expression suggests that oxytocin acts similarly in males and females in key regions of the circuitry underlying cocaine seeking.
Systemic oxytocin activated oxytocin cell bodies within the PVN, as indexed by Fos+ oxytocin neurons, consistent with a previous finding by Carson et al. (2010). Relatively small amounts of oxytocin cross the blood brain barrier (Landgraf et al., 1979; Ermisch et al., 1985). It is believed that the small amounts of oxytocin initiate a feed-forward mechanism to propagate endogenous oxytocin production (Rossoni et al., 2008). Recently, Neumann and colleagues (2013) used microdialysis in rats to demonstrate that i.p. administration of oxytocin caused rapid peak levels in brain dialysates 30 minutes post injection, providing direct evidence that systemic oxytocin increases central oxytocin levels. Consistently, i.v. oxytocin (5 IU/kg) increased oxytocin levels in cerebral spinal fluid, peaking at 15 minutes post-dose and gradually returning to baseline by 120 minutes in rhesus macaques (Freeman et al., 2016). However, it remains unclear how peripheral administration of oxytocin propagates central oxytocin function. Lee et al. (2017) demonstrated that oxytocin administered through intranasal and i.v. administration increased oxytocin in cerebral spinal fluid, but did not activate a feed forward mechanism to elevate endogenous oxytocin.
Our findings offer indirect evidence of oxytocin penetration of the blood brain barrier and are consistent with previous studies showing that systemic oxytocin results in changes in Fos+ oxytocin neurons (Carson et al., 2010b) and normalizing drug and drug-cued increases in Fos expression (Carson et al., 2010b; Cox et al., 2017). Further, increased Fos+ oxytocin neurons in the PVN cannot be attributed to the reexposure to cocaine-associated cues, as no differences were seen between the extinction and the cue test conditions and are, therefore, more likely the result of oxytocin administration. In brain areas where the conditioned cue increased Fos+ cells relative to the Ext condition, oxytocin brought the number of Fos+ cells down to extinction levels. Combined, these data show that oxytocin can act in a regionally specific manner to induce or ameliorate neuronal activation.
We previously demonstrated that systemic oxytocin administration suppressed cocaine-induced and basal locomotor activity to a greater extent in females relative to males (Zhou et al., 2015; Leong et al., 2016). We hypothesized that suppressed locomotion in females, but not males, was possibly due to more pronounced peripheral effects in females, such as uterine contraction (Tahara et al., 2002). Thus, the question remained whether oxytocin effects on drug-cued reinstatement were peripherally vs centrally driven in males and females. Here we show that central and peripheral administration of oxytocin produced a similar attenuation of cue-induced cocaine seeking in males and females providing strong evidence that oxytocin’s effects on cocaine seeking occur through central mechanisms in both sexes.
Interestingly, males showed a higher number of oxytocin cell bodies in the PVN relative to females. This finding does not correspond with previous findings showing that PVN oxytocin-immunoreactive neurons were higher in female mice (Haussler et al., 1990), mandarin voles (Qiao et al., 2014), and Brandt’s voles (Xu et al., 2010). However, a lack of sex differences in PVN oxytocin-immunoreactive neurons has been seen in prairie voles (Wang et al., 1996) and naked mole rats (Rosen et al., 2008). Additionally, oxytocin mRNA in Wistar rats was found to be similar in males and females (Dumais et al., 2013). The inconsistency of these findings suggests that oxytocin cell body expression in the PVN may differ based on species and also be dependent on the method of quantification. Albeit speculative, it is possible that a history of cocaine self-administration and/or extinction may alter expression of oxytocin cell bodies in this region in a sex-specific manner. Support for this notion comes from studies showing that central administration of the cocaine-amphetamine-regulated transcript induces Fos expression in PVN oxytocin cells (Vrang et al., 2000) and meth increased oxytocin mRNA expression in the NAc in male rats (Cadet et al., 2014). Whether these changes vary as a function of sex needs to be determined.
As previously reported (Carson et al., 2010b), oxytocin increased Fos expression in the CeA. The CeA receives direct projections from oxytocin cell bodies in the PVN and supraoptic nucleus (Knobloch et al., 2012). These projections have been found to drive oxytocin’s attenuation of fear-conditioned stimuli (Ebner et al., 2005; Knobloch et al., 2012). Further, reexposure to cocaine-associated cues following a period of abstinence resulted in robust drug seeking accompanied by increased ERK-activation in CeA, and blocking ERK phosphorylation decreased drug seeking (Lu et al., 2005). Additionally, pharmacological inhibition of the CeA attenuated conditioned cue-induced reinstatement of cocaine seeking (Kruzich and See, 2001). In mice CeA oxytocin receptor binding was increased during cocaine self-administration, cued, and primed reinstatement (Georgiou et al., 2015) as well as during noncontingent cocaine exposure and chronic withdrawal (Georgiou et al., 2016). However, our current results showed that oxytocin potentiated CeA Fos expression beyond that of cue exposure only. Although Fos+ cells in the Cue+Veh condition were increased relative to the extinction condition, this increase did not reach statistical significance on posthoc comparisons. As such, it is unclear whether oxytocin-induced Fos expression in the CeA was due to administration of the peptide alone or in combination with cocaine paired cues.
Oxytocin normalized cocaine cue-induced Fos expression in both the mPFC and NAc. The NAc is especially important in mediating the maladaptive consequences of cocaine addiction (Kalivas, 2009). The mPFC mediates inhibitory control and is impaired following extended drug use (Jentsch and Taylor, 1999). Glutamatergic corticostriatal projections have been extensively implicated in the neurocircuitry of addiction (Koob and Volkow, 2016), and inhibition of the nucleus accumbens core, but not the shell, abolishes cocaine cued reinstatement (Fuchs et al., 2004). It has also been shown that optogenetic inhibition of the prelimbic mPFC-NAc pathway reduces cued reinstatement following extinction (Stefanik et al., 2016). Interestingly, oxytocin also normalizes meth-induced Fos activation in these same brain areas (Carson et al., 2010b) and p-ERK levels in the mPFC during cocaine-primed reinstatement in rats (Zhou et al., 2015). One possible mechanism underlying this normalization may be through activation of oxytocin receptors localized on GABAergic interneurons in the mPFC (Nakajima et al., 2014) and NAc (Dölen et al., 2013), in a manner similar to that reported for oxytocin’s mechanism in the CA1 region of the hippocampus (Zaninetti and Raggenbass, 2000; Owen et al., 2013). In the hippocampus, oxytocin activates receptors on fast-spiking interneurons, which in turn deliver a millisecond-delayed inhibitory postsynaptic potential (i.e., feed forward inhibition) ultimately resulting in suppression of spontaneous pyramidal cell firing (Owen et al., 2013).
Oxytocin also normalized cue-induced Fos (current report) and meth-induced Fos activation in the STN (Carson et al., 2010b). The STN processes and encodes natural and drug reward (Baunez et al., 2005; Lardeux et al., 2009). Recent studies have shown that STN inactivation attenuated cued reinstatement (Bentzley and Aston-Jones, 2016), and site-specific oxytocin infusions into the STN blocked methamphetamine-conditioned place preference and primed reinstatement (Baracz et al., 2015; Baracz and Cornish, 2016). However, the specific mechanisms by which oxytocin attenuates drug-conditioned behaviors in the STN remain to be determined and may also be subject to the localization of oxytocin receptors within this structure. Additional studies are required to identify cell-type specificity of oxytocin receptors in the STN.
As mentioned earlier, sexual dimorphisms have been characterized in the peripheral and central oxytocin systems (Haussler et al., 1990; Dumais et al., 2013), as well as in the formation, maintenance, and relapse of cocaine addiction (for review, see Becker and Koob, 2016). In our previous reports, oxytocin decreased cued cocaine seeking in male (Zhou et al., 2015) and female rats (Leong et al., 2016). Here, we report direct comparisons of data collected from both sexes that were conducted simultaneously under identical conditions. The only sex difference we found was that females self-administered more cocaine than males when adjusted for body weight. Females have been reported to show greater cocaine-primed reinstatement than males (Lynch and Carroll, 2000; Kippin et al., 2005; Anker and Carroll, 2011), but cue-induced reinstatement is relatively similar between sexes (Fuchs et al., 2005; Feltenstein et al., 2011). Previously, Feltenstein et al. (2011) reported no sex differences in cocaine-cued reinstatement as well as similar responding across all estrous cycle phases. In light of these earlier findings, all rats underwent the cued reinstatement tests according to response criteria. A recent review (Becker and Koob, 2016) suggested that most hormonal effects occur during acquisition of drug taking and less so once compulsive drug taking has been established. It is possible, however, that oxytocin may interact with cycle phase during reinstatement testing even though cycle does not influence reinstated cued cocaine seeking. This was not the case in response to meth-primed reinstatement, in which oxytocin decreased reinstatement seeking regardless of cycle phase (Cox et al., 2013).
Despite the existence of sexual dimorphisms in central and peripheral oxytocin receptor distribution and function (Vane and Williams, 1973; Adan et al., 1995; Dumais et al., 2013), and inherent sex differences in cocaine addiction (Becker and Koob, 2016), we observed sex similarities at behavioral and cellular levels. In fact, our findings suggest that oxytocin is equally effective at attenuating cocaine-seeking behavior in males and females and, furthermore, appears to facilitate similar mechanisms within similar brain regions.
Statement of Interest
None
Supplementary Material
Acknowledgments
We thank Inasia Brown, Courtney F. Moody, and Maggie Wasacz for technical assistance. Cocaine was supplied by NIDA.
This work was supported by National Institutes of Health/National Institute of Drug Addiction (P50DA016511 and DA033049 to C.M.R., T32DA728823 to K.C.L., and HD055885 to L.R.F.).
References
- Adan RA, Van Leeuwen FW, Sonnemans MA, Brouns M, Hoffman G, Verbalis JG, Burbach JP (1995) Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: partial sequence and immunocytochemical localization. Endocrinology 136:4022–4028. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. In: Biological Basis of Sex Differences in Psychopharmacology,pp. 73–96. Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Cornish JL (2016) The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Front Neuroendocrinol. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Parker LM, Suraev AS, Everett NA, Goodchild AK, McGregor IS, Cornish JL (2015) Chronic methamphetamine self-administration dysregulates oxytocin plasma levels and oxytocin receptor fibre density in the nucleus accumbens core and subthalamic nucleus of the rat. Journal of Neuroendocrinology 28. [DOI] [PubMed] [Google Scholar]
- Baunez C, Dias C, Cador M, Amalric M (2005) The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nat Neurosci 8:484–489. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex differences in animal models: focus on addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G (2016) Inhibiting subthalamic nucleus decreases cocaine demand and relapse: therapeutic potential. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE (2012) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Ladenheim B, McCoy MT, Krasnova IN, Lehrmann E, Jayanthi S (2014) Enhanced upregulation of CRH mRNA expression in the nucleus accumbens of male rats after a second injection of methamphetamine given thirty days later. PloS One 9:e84665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS (2010a) Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology 58:38–43. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, Boucher AA, McGregor IS (2010b) Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Bio 15:448–463. [DOI] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology 38:2343–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G. (2017) Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol Psychiatry 81:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Darvishzadeh A, Huang KW, Malenka RC (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013) Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav 64:693–701. [DOI] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Krömer SA, Singewald N, Neumann ID (2005) Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology 30:223–230. [DOI] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Rühle HJ, Skopkova J,Hrbas P, Landgraf R (1985) On the blood-brain barrier to peptides: accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinol Exp 19:29–37. [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2006) Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behavioural brain research 174:1–8. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE (2011) Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 216:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, Roberts JA (2016) Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology 66:185–194. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE (2004) Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 176:459–465. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM et al. (2005) Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 179:662–672. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE (2006) The role of the basolateral amygdala in stimulus–reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. European Journal of Neuroscience 23:2809–2813. [DOI] [PubMed] [Google Scholar]
- George P, Charles W (2007) The rat brain in stereotaxic coordinates. Qingchuan Zhuge translate, 32. Beijing: People’s Medical Publishing House. [Google Scholar]
- Georgiou P, Zanos P, Ehteramyan M, Hourani S, Kitchen I, Maldonado R, Bailey A (2015) Differential regulation of mGlu5R and ΜOPr by priming‐and cue‐induced reinstatement of cocaine‐seeking behaviour in mice. Addiction Biology 20:902–912. [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Garcia-Carmona JA, Hourani S, Kitchen I, Kieffer B, Bailey A (2015) The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: involvement of dopaminergic, noradrenergic and MOPr systems. European Neuropsychopharmacology 25:2459–2464. [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Hourani S, Kitchen I, Bailey A (2016) Cocaine abstinence induces emotional impairment and brain region‐specific upregulation of the oxytocin receptor binding. European Journal of Neuroscience 44:2446–2454. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81:629–683. [DOI] [PubMed] [Google Scholar]
- Haussler HU, Jirikowski GF, Caldwell JD (1990). Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J Chem Neuroanat 3:271–276. [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF (1999) Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus: size changes in relation to age and sex. J Clin Endocrinol Metab 84:4637–4644. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. (2009) The glutamate homeostasis hypothesis of addiction. Nature Publishing Group 10:561–572. [DOI] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC (2017) Oxytocin reduces ethanol self-administration in mice. Alcohol Clin Exp Res 41:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182:245–252. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73:553–566. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GL, Horváth Z, Sarnyai Z, Faludi M, Telegdy G (1985) Oxytocin and a C-terminal derivative (Z-prolyl-D-leucine) attenuate tolerance to and dependence on morphine and interact with dopaminergic neurotransmission in the mouse brain. Neuropharmacology 24:413–419. [DOI] [PubMed] [Google Scholar]
- Kovács GL, Borthaiser Z, Telegdy G (1985) Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sci 37:17–26. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE (2001) Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci 21:Rc155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Ermisch A, Hess J (1979) Indications for a brain uptake of labelled vasopressin and ocytocin and the problem of the blood-brain barrier. Endokrinologie 73:77–81. [PubMed] [Google Scholar]
- Lardeux S, Pernaud R, Paleressompoulle D, Baunez C (2009) Beyond the reward pathway: coding reward magnitude and error in the rat subthalamic nucleus. J Neurophysiol 102:2526–2537. [DOI] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM (2016) Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp Clin Psychopharmacol 24:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005) Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8:212–219. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 148:196–200. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A (2007) Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci 26:1026–1035. [DOI] [PubMed] [Google Scholar]
- Morales-Rivera A, Hernández-Burgos MM, Martínez-Rivera A, Pérez-Colón J, Rivera R, Montalvo J, Rodriguez-Borrero E, Maldonado-Vlaar CS (2014) Anxiolytic effects of oxytocin in cue-induced cocaine seeking behavior in rats. Psychopharmacology 231:4145–4155. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Gorlich A, Heintz N (2014) Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, . Landgraf R (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38:1985–1993. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW (2013) Oxytocin enhancement of CA1 spike transmission by modulation of fast-spiking interneurons. Nature 500:458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF (2009) Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology 56:856–865. [DOI] [PubMed] [Google Scholar]
- Qiao X, Yan Y, Tai F, Wu R, Hao P, Fang Q, Zhang S (2014) Levels of central oxytocin and glucocorticoid receptor and serum adrenocorticotropic hormone and corticosterone in mandarin voles with different levels of sociability. Behav Brain Res 274:226–234. [DOI] [PubMed] [Google Scholar]
- Reichel CM, See RE (2012) Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. International Journal of Neuropsychopharmacology 15:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GJ, de Vries GJ, Goldman SL, Goldman BD et al. (2008) Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience 155:809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F (2008) Emergent synchronous bursting of oxytocin neuronal network. PLoS Comput Biol 4:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G (1991) Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites. Neuropeptides 19:51–56. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL (1994) Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology 19:85–117. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL (2014) Oxytocin in learning and addiction: from early discoveries to the present. Pharmacol Biochem Behav 119:3–9. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW (2016) Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct Funct 221:1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara M, Morishige KI, Sawada K, Ikebuchi Y, Kawagishi R, Tasaka K, Murata Y (2002) RhoA/Rho-kinase cascade is involved in oxytocin-induced rat uterine contraction. Endocrinology 143:920–929. [DOI] [PubMed] [Google Scholar]
- Vane JR, Williams KI (1973) The contribution of prostaglandin production to contractions of the isolated uterus of the rat. Br J Pharmacol 48:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pan Y, Wang Z, Zhang Z (2013) Species differences in the immunoreactive expression of oxytocin, vasopressin, tyrosine hydroxylase and estrogen receptor alpha in the brain of Mongolian gerbils (Meriones unguiculatus) and Chinese striped hamsters (Cricetulus barabensis). PLoS One 8:e65807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR (1996) Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol 366:726–737. [DOI] [PubMed] [Google Scholar]
- Xu L, Pan Y, Young KA, Wang Z, Zhang Z (2010) Oxytocin and vasopressin immunoreactive staining in the brains of Brandt’s voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton). Neuroscience 169:1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M (2000) Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 141:794–801. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, Raggenbass M (2000) Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. Eur J Neurosci 12:3975–3984. [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A (2014) The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology 39:855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pruitt C, Shin CB, Garcia AD, Zavala AR, See RE (2014) Fos expression induced by cocaine-conditioned cues in male and female rats. Brain Struct Funct 219:1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sun WL, Young AB, Lee K, McGinty JF, See RE (2015) Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.