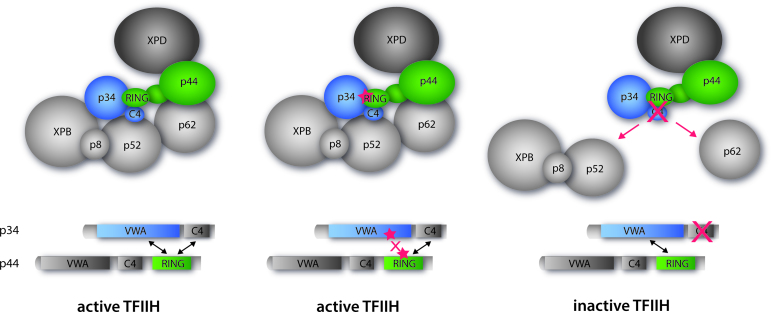
Figure 5.
Cartoon representation depicting the summary of our observations with the same coloring scheme as used in Figure 1 showing core-TFIIH with XPD. Left panel: wild-type active TFIIH. Middle panel: TFIIH mutated within the minimal p34/p44 interface (structurally characterized in this study) maintaining the active complex. Right panel: TFIIH with the C4 domain of p34 missing. This p34 variant still interacts with p44 but other vital interactions are missing leading to an inactive TFIIH.