Abstract
Production of reactive oxygen and nitrogen species (ROS), such as hydrogen peroxide, superoxide and hydroxyl radicals, has been linked to cancer, and these oxidative molecules can damage DNA. Base excision repair (BER), a major repair system maintaining genome stability over a lifespan, has an important role in repairing oxidatively induced DNA damage. Failure of BER leads to toxic consequences in ROS-exposed cells, and ultimately can contribute to the pathobiology of disease. In our previous report, we demonstrated that oxidized nucleotide insertion by DNA polymerase β (pol β) impairs BER due to ligation failure and leads to formation of a cytotoxic repair intermediate. Biochemical and cytotoxic effects of ligation failure could mediate genome stability and influence cancer therapeutics. In this review, we discuss the importance of coordination between pol β and DNA ligase I during BER, and how this could be a fundamental mechanism underlying human diseases such as cancer and neurodegeneration. A summary of this work was presented in a symposium at the International Congress of Radiation Research 2015 in Kyoto, Japan.
Keywords: genome stability, oxidative DNA damage, base excision repair, DNA polymerase β, DNA ligase I
INTRODUCTION
The cell is continually exposed to hazards that change the chemical structure of the genomic DNA and reduce its stability, promoting deleterious human health consequences [1, 2]. Oxidative stress produced by reactive oxygen and nitrogen species (ROS) has been linked to biochemical pathways of cancer initiation and promotion [3, 4]. A high level of ROS in carcinoma cells compared with in healthy tissues has been reported in many studies [5]. The deoxynucleoside triphosphates (dNTPs) in the nucleotide pool are vulnerable to oxidation by ROS, leading to oxidized forms of these DNA precursors [6]. The 8-oxo-2′-deoxyguanosine (8-oxodG) and 8-oxo-2′-deoxyguanosine-5′-triphosphate (8-oxodGTP) are major products of oxidized forms of deoxyguanosine (dG) and deoxyguanosine triphosphate (dGTP), respectively [7]. Both lesions are known as markers of oxidative stress in different cancer types [8, 9]. There are many reports describing elevated and altered levels of oxidation products of purine or pyrimidine bases in a plethora of ageing-related diseases, such as cancer [7, 10]. For example, an increase in the ratio of 8-oxodG monophosphate (8-oxodGMP) to 8-oxodGTP in the nucleotide pool has been reported in cancer cells, such as in breast carcinoma [11, 12]. The deleterious effects of oxidized dNTPs stem from their incorporation into DNA during DNA replication and repair [13].
The small single-base DNA lesions, such as apurinic/apyrimidinic (AP)-sites and DNA damage from oxidation, are repaired by the base excision repair (BER) pathway [14]. BER protects cells against cancer, ageing and neurodegeneration [15]. One of the multiple subpathways of BER, termed ‘single-nucleotide BER’, requires the coordinated efforts of three core BER enzymes in mammalian systems: apurinic/apyrimidinic 1 endonuclease (APE1), DNA polymerase β (pol β), and DNA ligase I [16]. Biochemical studies with purified human BER enzymes revealed a specialized type of coordination between these enzymes [17]. During BER, DNA substrates and reaction products are channeled from one step to the next in a sequential fashion, so that release of toxic BER intermediates is minimized [18]. This includes a hand-off of a DNA repair intermediate after the pol β DNA synthesis (‘gap filling’) step to the DNA ligation step during BER [19]. If the ligation step fails, a blocked 5′-adenylated BER intermediate can accumulate in cells. It has been found that coordination between these BER enzymes is crucial and, when deficient, is associated with loss of cell viability and genomic stability [17, 19]. However, the mechanism of how unrepaired single-strand break intermediates are generated during the repair of endogenously and environmentally induced cytotoxic and mutagenic lesions is still under investigation [20, 21]. Left unrepaired, these blocked ends could interrupt BER, block replication and transcription, and be converted into toxic double-stranded breaks. A reduction of these intermediates is important, since they are seen as at-risk structures that could be more toxic than the initial DNA lesions such as AP-sites. Therefore, failure to the repair of these intermediates could be a fundamental mechanism underlying human diseases including those related to irradiation exposure.
In a previous study [22], we demonstrated that oxidized nucleotide insertion by pol β results in ligation failure and impairs BER. This ligation failure after insertion of an oxidized purine nucleotide corresponds to formation of the BER intermediate with blocked 3′-8-oxo-7,8-dihydro-2′-deoxyguanosine (3′-8-oxoG) and 5′-adenylated, (5′-AMP)–containing ends. Furthermore, we found ligation failure differences depending both on the type of oxidized nucleotide that pol β inserted into a single-nucleotide gapped DNA substrate and the identity of the template base (dG, dC, dT or dA) against which pol β inserted an oxidized purine nucleotide. This appears to reflect a functional interaction between these two BER enzymes, pol β and DNA ligase I, as they bind and interact on the BER intermediate. In this review, we discuss these differences in failed ligation. We show gel images from biochemical experiments that illustrate the products of pol β oxidized nucleotide insertion and ligation by DNA ligase I, along with ligation failure, depending on the type of oxidized purine nucleotide inserted and the identity of the template base as a function of time of incubation.
Furthermore, in a previous study [22], we observed more cytotoxicity and DNA strand breaks in mouse embryonic fibroblast cells expressing pol β than in pol β null cells. Our in vitro findings using cell extracts demonstrated that there is a limited role of DNA end-processing enzymes in the repair of impaired BER intermediates, after pol β oxidized nucleotide insertion and ligation failure. These results point to the accumulation of repair intermediates with single-strand breaks. Overall, the results suggest that a DNA strand break with an oxidized purine base at the 3′-margin leads to an oxidative stress–induced stalled repair intermediate that is capable of triggering cell death. This could be a component of a fundamental mechanism linking genomic instability to human diseases that are associated with oxidative stress, such as cancer and neurodegeneration.
MATERIALS AND METHODS
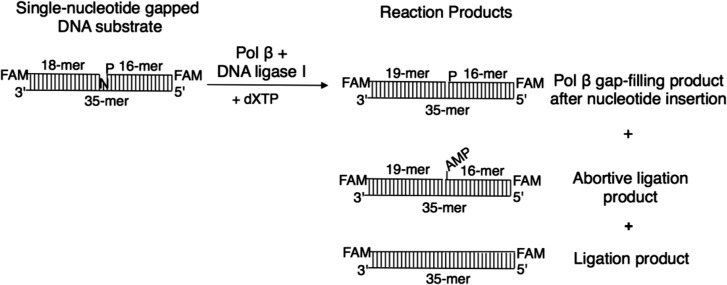
The materials and methods used to obtain the experimental results shown here have been previously reported [22]. The reactions were performed under steady-state conditions, where the DNA substrate was in excess relative to the enzymes, i.e. including pol β and DNA ligase I. Figure 1 presents the DNA substrate and reaction products illustrated in Figs 2–4.
Fig. 1.
The DNA substrate and reaction products observed in the coupled BER assay. FAM indicates the presence of a fluorescent tag; N in the single-nucleotide gapped DNA substrate represents a template base (A, T, G or C), and dXTP is the oxidized nucleotide (8-oxodGTP, 8-oxodATP or 2-OH-dATP).
Fig. 2.
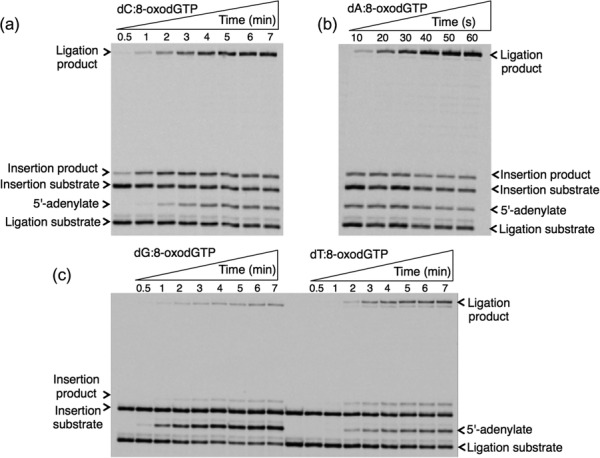
Template-base–dependent differences in ligation after pol β 8-oxodGTP insertion. Gel images showing the products for oxidized nucleotide insertion, ligation and ligation failure after 8-oxodGTP insertion against template base C (a), A (b), G (c) and T (d). We previously published the gel image that shows the products for insertion, ligation, and abortive ligation for 8-oxodGMP insertion against template base C [22]. The illustration in Panel a is the result of a repeat experiment.
Fig. 4.
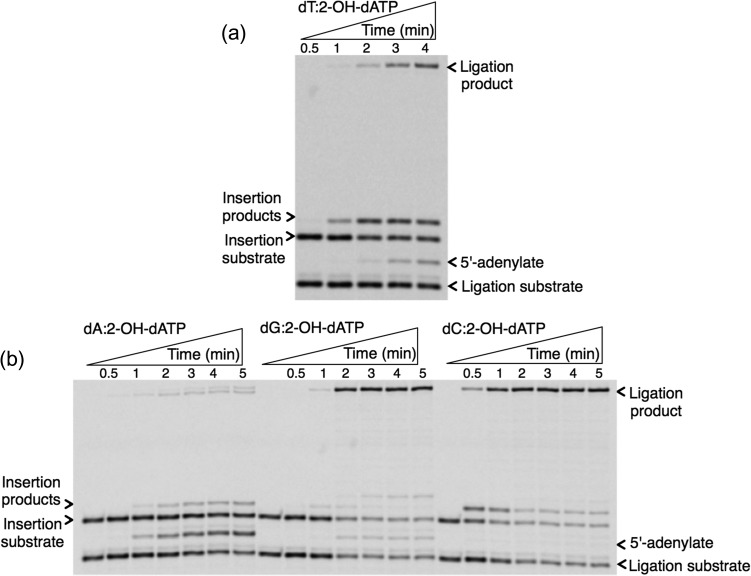
Template-base–dependent differences in ligation after pol β 2-OH-dATP insertion. Gel images showing the products for oxidized nucleotide insertion, ligation and ligation failure after 2-OH-dATP insertion against template base T (a), and C, A or G (b).
RESULTS AND DISCUSSION
In a previous study, we were able to measure pol β nucleotide insertion into a single-nucleotide gapped DNA substrate with template bases dA, dG, dC or dT, and ligation reactions in the same reaction mixture, as a function of time [22]. This biochemical approach enabled us to compare various products of ligation failure (i.e. 5′-adenylate formation). Here, we show the products for oxidized nucleotide insertion, ligation and ligation failure in the same gel images (Fig. 1) and discuss differences in these products, depending on the template-base against which pol β inserts an oxidized purine nucleotide.
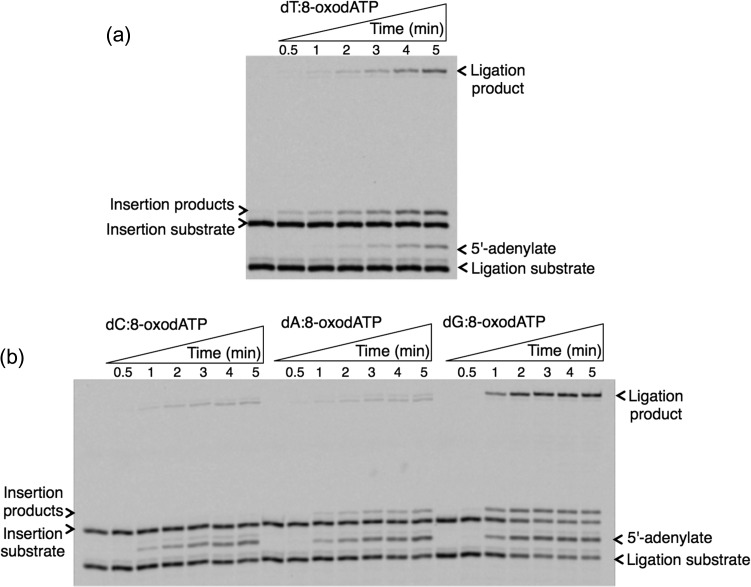
For the oxidized guanine nucleotide 8-oxodGTP (Fig. 2), the results show that pol β can insert 8-oxodGTP more efficiently against template bases dC and dA, accordingly, while the products for insertion against template bases dG and dT are weaker. On the other hand, more ligation products were observed for template bases dC and dA, while ligation failure was in the order of dG ≥ dA > dC ≈ dT. Interestingly, we observed ligation failure products at very early time points of the reaction (i.e. 10 s), only after pol β 8-oxodGMP insertion against template base dA. This difference could be due to the glycosidic bond conformation preference that 8-oxodGTP has, depending on the identity of the template base: 8-oxodGMP (anti):dC (anti) versus 8-oxodGMP (syn):dA (anti). This conformational difference that pol β shows after 8-oxodGMP insertion against template base dC versus dA could affect its functional interaction with DNA ligase I on the BER intermediate and the fate of the DNA ligation reaction. For the oxidized adenine nucleotide (Fig. 3), 8-oxo-2′-deoxyadenosine-5′-triphosphate (8-oxodATP), the products for 8-oxodATP insertion are in the order dG ≥ dT ≥ dA > dC. Interestingly, the amount of ligation product is stronger for template base dG, while similar amounts of ligation failure products are observed for template bases dA, dC and dG. We also tested another oxidized form of dATP (Fig. 4), 2-hydroxydeoxyadenosine 5′-triphosphate (2-OH-dATP). The pol β template base preference for insertion of this oxidized nucleotide was in order of dT ≥ dC > dA > dG. This difference was observed in the face of ligation products that were much stronger after insertion against template bases dG and dC, involving successful conversion of inserted oxidized nucleotides into the ligation product. For the template base dT, even where there was more insertion by pol β, DNA ligase was unable to handle the new base pair and complete ligation. In addition, for template base dA, where pol β did not efficiently insert 2-OH-dATP, we observed more ligation failure. These results indicate a functional interaction or crosstalk between pol β and DNA ligase I, where the enzymes are responding to structural differences in the BER intermediate during channeling of the nicked intermediate from one step to the next in the repair pathway.
Fig. 3.
Template-base–dependent differences in ligation after pol β 8-oxodATP insertion. Gel images showing the products for oxidized nucleotide insertion, ligation and ligation failure after 8-oxodATP insertion against template base T (a), and C, A or G (b).
Furthermore, in a previous study [22], we also showed that 3′-DNA end processing enzymes, i.e. OGG1, NEIL1, NTH1, APE1 and Tdp1, had very limited activity in the repair of the BER intermediate with blocked 3′-8-oxoG and 5′-AMP-containing ends. This result indicates the persistence of the 3′-end lesion on the BER intermediate following pol β oxidized nucleotide insertion, which could lead to more DNA strand breaks.
Overall, these results demonstrate that the structure or sequence identity in the BER intermediate to which both pol β and DNA ligase I bind has an impact on the success or failure of the ligation reaction that could influence fate of the BER pathway. In order to understand the functional interaction between these BER enzymes better, additional studies, i.e. structural studies combining both enzymes with an incoming oxidized nucleotide, are required. Moreover, one of the key areas that has not been studied is how the structural adjustments that DNA polymerases exhibit after nucleotide insertion could impact downstream repair steps and substrate channeling of DNA intermediates in the repair pathway. In addition to structural studies, further biochemical experiments to examine the effect of different DNA structures (i.e. base substitution or frameshift intermediates in the template strand, or mismatches at the primer terminus) in the coordination between gap-filling and nick-ligation steps during the BER pathway are also needed. In our previous study [22], we reported more oxidative stress–induced cell killing effect in mouse embryonic cells expressing pol β, as well as increased cytotoxicity in the cells with MTH1, oxidized dNTP hydrolyase, and gene deletion. To better understand the pol β oxidized nucleotide insertion–dependent effect on cytotoxicity, oxidized or modified dNTP pool measurements from these cells are necessary. The modulation of oxidized nucleotide pool size by targeting BER proteins could be a promising approach for the future of personalized therapy for cancer.
ACKNOWLEDGEMENTS
This work was supported by the Division of Intramural Research of the National Institute of Environmental Health Sciences (NIEHS) and the National Institutes of Health (NIH) [grant numbers Z01-ES050158 and Z01-ES050159 to S.H.W.], and M.Ç. is also funded by 1K99-ES026191-01 (NIH/NIEHS).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) and the National Institutes of Health (NIH) (grant numbers Z01-ES050158 and Z01-ES050159).
REFERENCES
- 1. Lutz WK. Endogenous genotoxic agents and processes as a basis of spontaneous carcinogenesis. Mutat Res 1990;238:287–95. [DOI] [PubMed] [Google Scholar]
- 2. Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res 1991;250:3–16. [DOI] [PubMed] [Google Scholar]
- 3. Reuter S, Gupta SC, Chaturvedi MM et al. . Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valavanidis A, Vlachogianni T, Flotakis C et al. . Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health 2013;10:3886–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS 2007;115:81–103. [DOI] [PubMed] [Google Scholar]
- 6. Rai P. Oxidation in the nucleotide pool, the DNA damage response and cellular senescence: defective bricks build a defective house. Mutat Res 2010;703:71–81. [DOI] [PubMed] [Google Scholar]
- 7. Nakabeppu Y, Sakumi K, Sakamoto K et al. . Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. J Biol Chem 2006;387:373–9. [DOI] [PubMed] [Google Scholar]
- 8. Roszkowski K, Jozwicki W, Blaszczyk P et al. . Oxidative damage DNA: 8-oxoGua and 8-oxodG as molecular markers of cancer. Med Sci Monit 2011;17:CR329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valavanidis A, Vlachogianni T, Flotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:120–39. [DOI] [PubMed] [Google Scholar]
- 10. Ventura I, Russo MT, De Luca G et al. . Oxidized purine nucleotides, genome instability and neurodegeneration. Mutat Res 2010;703:59–65. [DOI] [PubMed] [Google Scholar]
- 11. Hah SS, Mundt JM, Kim HM et al. . Measurement of 7,8-dihydro-8-oxo-2-deoxyguanosine metabolism in MCF-7 cells at low concentrations using accelerator mass spectrometry. Proc Natl Acad Sci U S A 2007;104:11203–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sova H, Jukkola-Vuorinen A, Puistola U et al. . 8-Hydroxydeoxyguanosine: a new potential independent prognostic factor in breast cancer. Br J Cancer 2010;102:1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katafuchi A, Nohmi T. DNA polymerases involved in the incorporation of oxidized nucleotides into DNA: their efficiency and template base preference. Mutat Res 2010;703:24–31. [DOI] [PubMed] [Google Scholar]
- 14. Lindahl T. Suppression of spontaneous mutagenesis in human cells by DNA base excision repair. Mutat Res 2000;462:129–35. [DOI] [PubMed] [Google Scholar]
- 15. Pan MR, Li K, Lin SY et al. . Connecting the dots: from DNA damage and repair to aging. Int J Mol Sci 2016;17:E685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srivastava DK, Berg BJ, Prasad R et al. . Mammalian abasic site base excision repair: identification of the reaction sequence and rate-determining steps. J Biol Chem 1998;273:21203–09. [DOI] [PubMed] [Google Scholar]
- 17. Prasad R, Shock DD, Beard WA et al. . Substrate channeling in mammalian base excision repair pathways: passing the baton. J Biol Chem 2010;285:40479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson SH, Kunkel TA. Passing the baton in base excision repair. Nat Struct Biol 2000;7:176–8. [DOI] [PubMed] [Google Scholar]
- 19. Prasad R, Singhal RK, Srivastava DK et al. . Specific interaction of DNA polymerase β and DNA ligase I in a multiprotein base excision repair complex from bovine testis. J Biol Chem 1996;271:16000–7. [DOI] [PubMed] [Google Scholar]
- 20. Freudenthal BD, Beard WA, Perera L et al. . Uncovering the polymerase-induced cytotoxicity of an oxidized nucleotide. Nature 2016;517:635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Çağlayan M, Wilson SH. Oxidant and environmental toxicant–induced effects compromise DNA ligation during base excision DNA repair. DNA Repair 2015;35:85–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Çağlayan M, Horton JK, Dai DP et al. . Oxidized nucleotide insertion by pol β confounds ligation during base excision repair. Nat Commun 2017;8:14045. [DOI] [PMC free article] [PubMed] [Google Scholar]