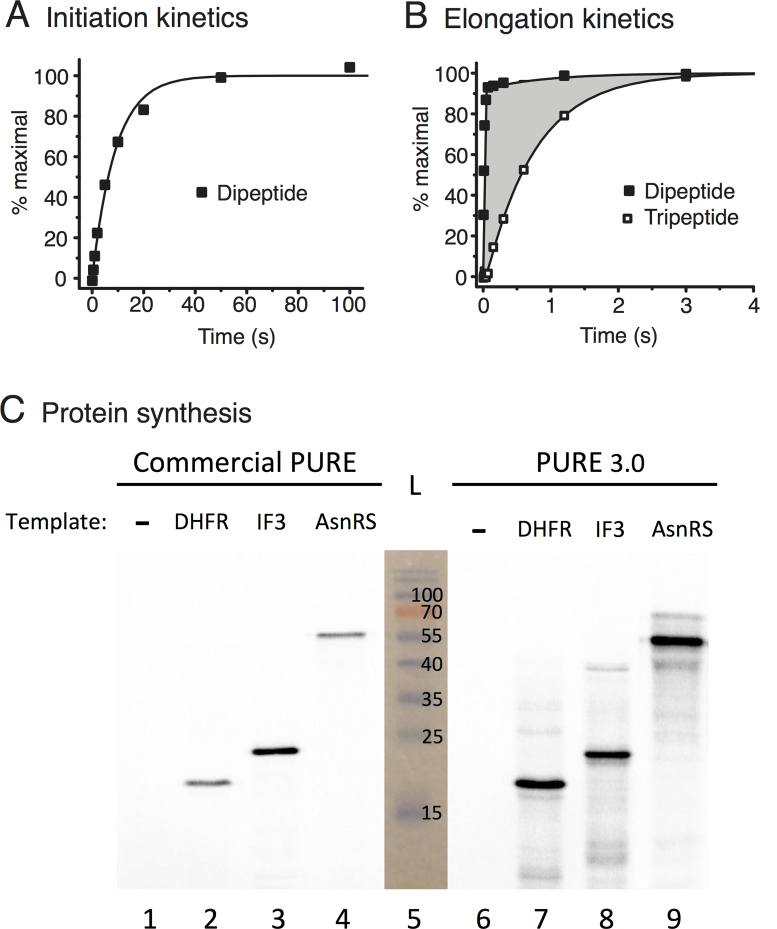
Figure 3.
Assay of purified proteins from pLD plasmids for kinetics of initiation, di- and tripeptide syntheses and for synthesis of full-length proteins (see Materials and Methods). (A) Rate-limiting splitting of vacant 70S ribosomes (initiation) monitored by time course of subsequent non-rate-limiting fMet-Phe dipeptide bond formation using proteins encoded by pLD2 and pLD3. (B) Elongation efficiency measured by templating synthesis of fMet-Phe-Phe tripeptide and monitoring formation of both dipeptide and tripeptide products using proteins encoded by pLD2 and pLD3. The shaded area is a measure of translocation mean time. (C) Comparison of full-length protein yields of DHFR, His6-IF3 and His6-AsnRS (predicted 18.0, 21.4 and 53.4 kDa, respectively) templated by uncut plasmids in a commercial PURE system versus our PURE 3.0 containing proteins encoded by pLD1, pLD2 and pLD3. Products were quantitated by incorporation of 35S-Met, separation by SDS-PAGE and phosphoimaging of the gel. A photograph of the pre-stained ladder (L) in lane 5 is superimposed, and the results are reproducible.