Figure 1.
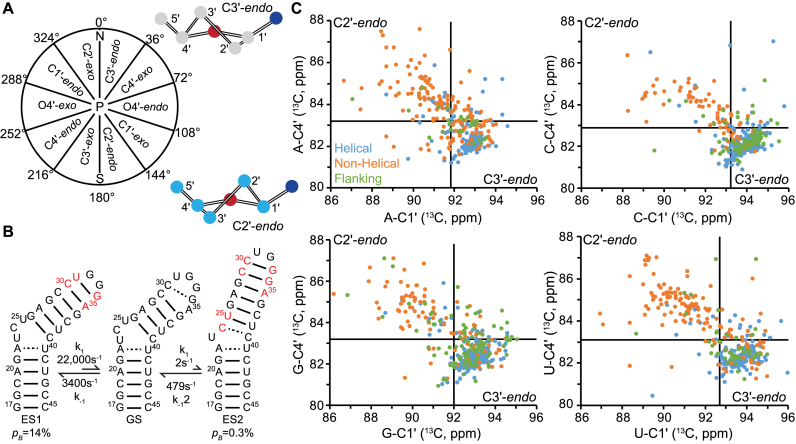
C1΄ and C4΄ chemical shifts as probes of sugar pucker. (A) The different sugar pucker conformations defined by a pseudorotation angle, P. (B) Chemical exchange between the TAR ground state (GS) and two excited states (ES1 and ES2). Shown are the populations (pB) and forward (k1) and reverse (k-1) rate constants obtained from Lee et al. (35). Nucleotides highlighted in red are expected to undergo sugar repuckering from C2΄-endo in the GS to C3΄-endo in ES1 or ES2 as they transition from non-helical to helical conformations. (C) C1΄ versus C4΄ chemical shift correlations obtained from the BMRB (54) for nucleotides classified as helical (blue), non-helical (orange) and flanking (green) based on the available RNA secondary structure. Plots shown for adenosine (N = 376), cytidine (N = 434), guanosine (N = 489) and uridine (N = 381). See Supplementary Figure S2 and Table S2 for chemical shift distributions. Solid lines indicate the upper boundaries for C3΄-endo chemical shift ranges as obtained based on the average and standard deviation of observed chemical shifts for helical nucleotides. The lower right quadrant represents the C3΄-endo sugar pucker; the upper left quadrant represents C2΄-endo pucker; the lower left quadrant likely represents C3΄-endo with low χ-angle (−130 ± 20°); and the upper right quadrant likely represents C3΄-endo with trans γ dihedral angle, or C2΄-endo with syn χ-angle.