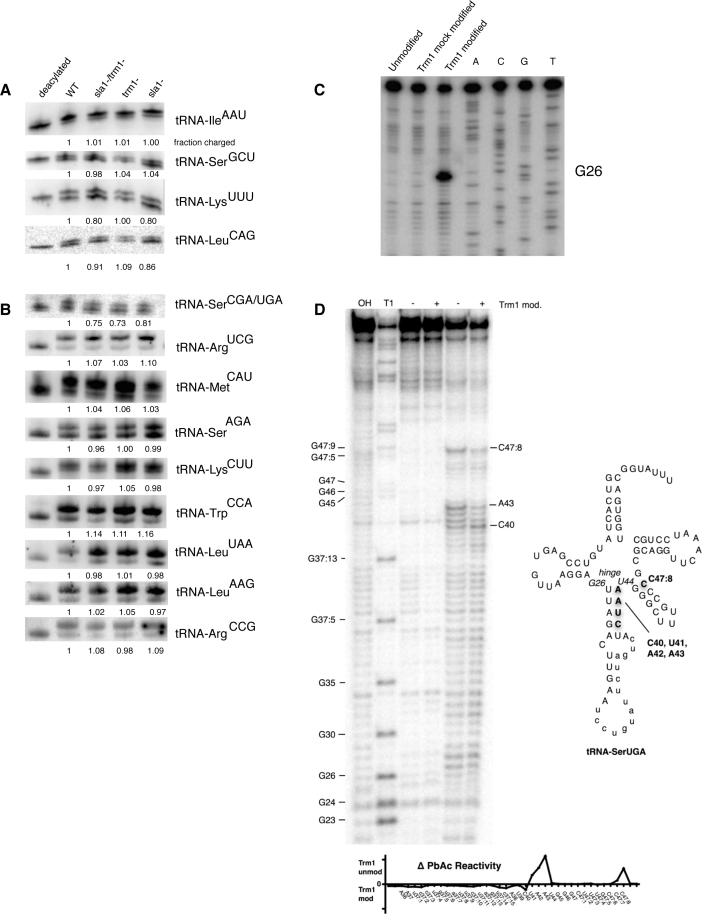
Figure 2.
Lack of Trm1p modification at G26 results in likely misfolding of tRNA-SerCGA/UGA. Acid-northern (A) and periodate oxidation/β-elimination (B) to assay charging levels of various tRNA species indicates a Sla1p/Trm1p associated tRNA charging defect for tRNA-SerCGA/UGA. (C) Reverse transcriptase primer extension of recombinant Trm1p-modified tRNA-PheGAA confirms specific modification of G26 in vitro. (D) Left: lead acetate chemical probing indicates altered structure in the anticodon stem loop and variable arm of pre-tRNA-SerUGA after in vitro modification with Trm1p. Right: secondary structure of tRNA-SerUGA. Nucleotides with altered chemical reactivity to lead acetate probing +/− Trm1p modification indicated in bold. Bottom: quantitated differential lead acetate reactivity +/− Trm1p modification.