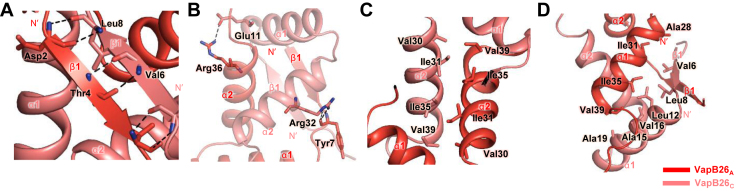
Figure 4.
Interfaces of the VapB26 dimer. More than 30 residues of each VapB26 N-terminal RHH DNA-binding domain participate in dimerization. Hydrogen bonds are shown as black dotted lines, and residues participating in hydrophobic interactions are shown as stick models. (A) Hydrogen bonding networks of antiparallel β-sheets. (B) Hydrogen bonds in the α1 and α2 helices. (C) Hydrophobic interaction in the α2 helices. (D) Range of hydrophobic forces from Ile35, Val39, Val6 and Leu8.