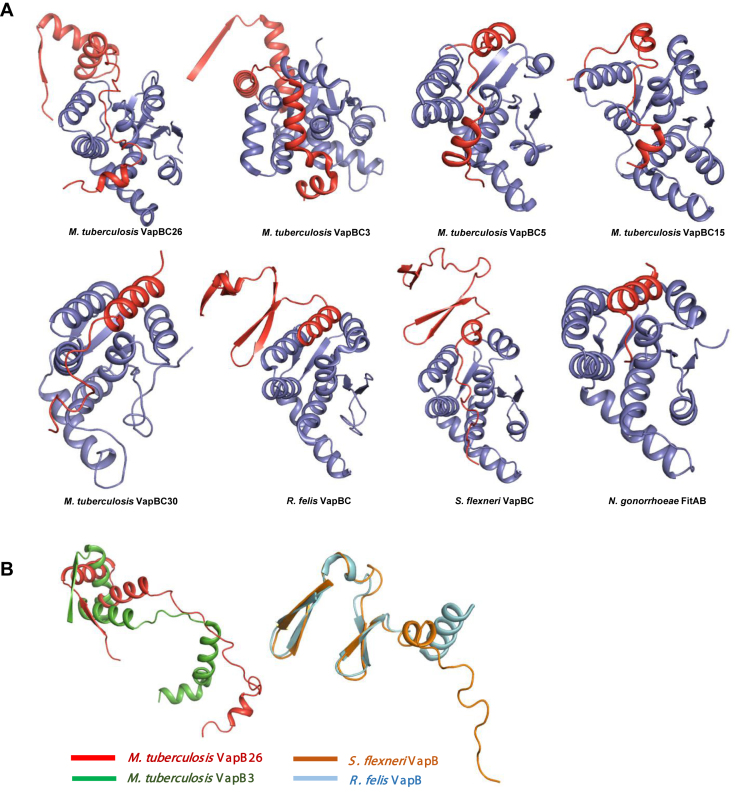
Figure 5.
Comparison of the structures of the VapBC complexes (A) and VapB antitoxins (B) with their homologs. (A) The complete structures of Mycobacterium tuberculosis VapBC3 and VapBC from Rickettsia felis and Shigella flexneri are shown; other known complexes lack a considerable moiety of the antitoxin. (B) VapB from R. felis and S. flexneri forms a β-barrel hairpin motif. VapB3 from M. tuberculosis forms an RHH N-terminal domain similar to that of VapB26, but the toxin-binding domain contains more α-helices than that of VapB26.