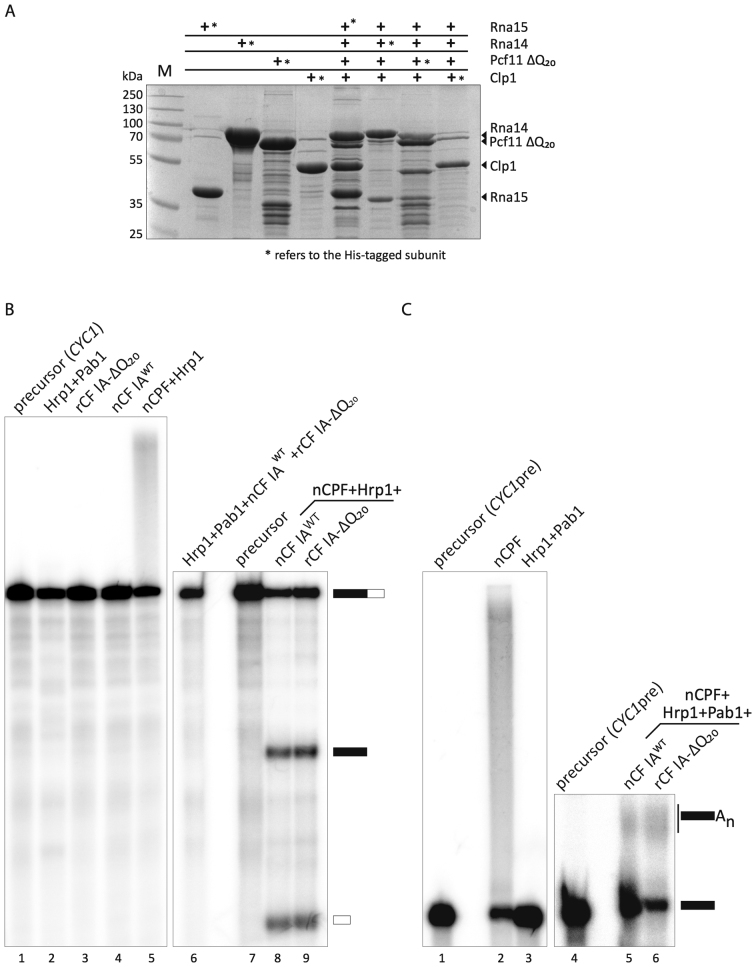
Figure 2.
Recombinant CF IA works efficiently in mRNA 3′-end formation. (A) CF IA was reconstituted by co-expression of the four CF IA subunits in E. coli. Each subunit was alternatively fused at its N-terminus with a hexahistidine tag allowing for protein purification. Pull-down experiments were performed from 10 ml cell culture after immobilization on affinity-resin. The bound proteins were eluted with imidazole and analyzed by a 10% SDS-PAGE. Proteins were revealed by Coomassie-blue staining. The asterisk (*) refers to the His-tagged subunit of CF IA. (B) In vitro cleavage assay with the CYC1 precursor was performed in the presence of native CPF (nCPF) and wild-type native CF IA (nCF IAWT) purified by TAP-tagging (lane 8) or recombinant CF IA (rCF IA-ΔQ20; lane 9). Hrp1 was included in the reaction to avoid cleavage at cryptic sites. Lanes 1 and 7: unreacted CYC1 precursor; lanes 1–6: negative controls showing the absence of cleavage activity of the different factors on their own. Lane 5 shows however the intrinsic and normal polyadenylation activity of Pap1-containing CPF. (C) In vitro polyadenylation assay of the CYC1 precleaved precursor (CYC1pre) with nCF IAWT (lane 5) or rCF IA-ΔQ20 (lane 6) and nCPF, Hrp1 and Pab1. The latter is required to control poly(A) tail elongation. Lanes 1 and 4: unreacted CYC1pre precursor; Lane 2: nCPF alone can polyadenylate unspecifically CYC1pre; lane 3: Hrp1 and Pab1 are inactive on their own.