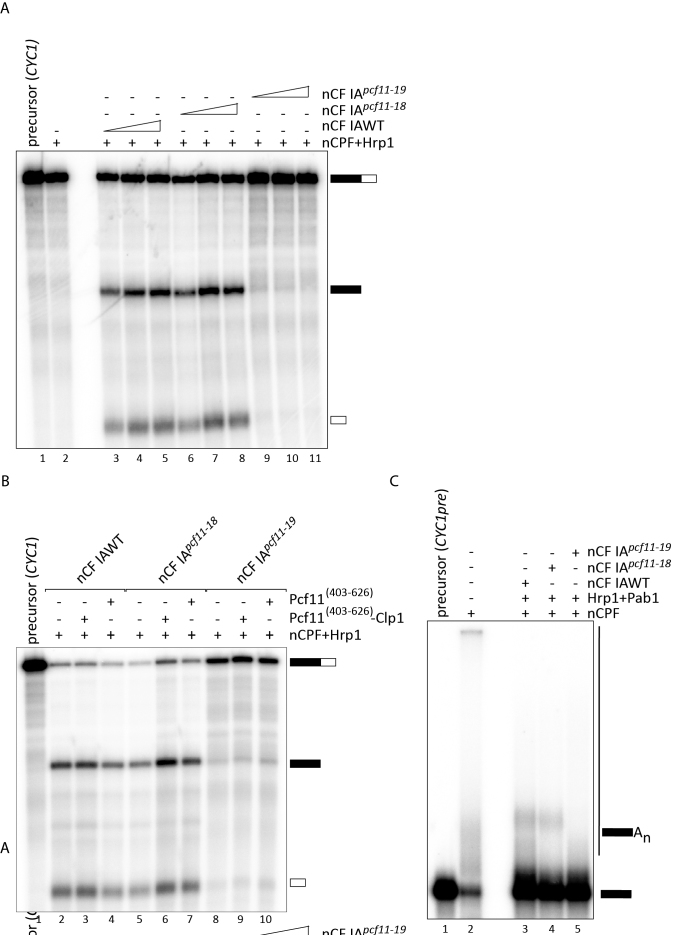
Figure 7.
Mutations in the zinc-binding motifs have distinct effects on 3′-end processing. (A) Pre-mRNA cleavage assay on the CYC1 precursor with TAP-purified factors from wild-type (nCF IAWT) and mutant (nCF IApcf11-18 and nCF IApcf11-19) cells. Lane 1: unreacted CYC1 precursor, lane 2: nCPF and Hrp1 were assayed as negative controls for the lack of cleavage activity in the absence of CF IA. Increasing amounts of nCF IAWT (0,5 μl, 1 μl and 2 μl; lanes 3–5) or nCF IApcf11-18 or nCF IApcf11-19 (2 μl, 4 μl and 8 μl lanes 6–8 and 9–11, respectively) were added to nCPF and Hrp1 to carry out the cleavage reaction. (B) Complementation of cleavage assays. Cleavage reactions were run as in (A) with 1 μl of nCF IAWT or 4 μl of nCF IApcf11-18 or nCF IApcf11-19. 100 ng of recombinant Pcf11[403-626]-Clp1 (lanes 3, 6, 9) or Pcf11[403-626] (lanes 4, 7, 10) were added to the reaction as indicated. Lane 1: unreacted CYC1 precursor. Lane 2: nCPF+Hrp1 were incubated with CYC1 as a negative control. (C) Polyadenylation assays performed on the CYC1 pre-cleaved precursor (CYC1pre) with the same amounts of nCF IAWT, nCF IApcf11-18 and nCF IApcf11-19 as in (B). Pab1 (140 ng) was added to the reaction to control de novo synthesis of poly(A) tails.