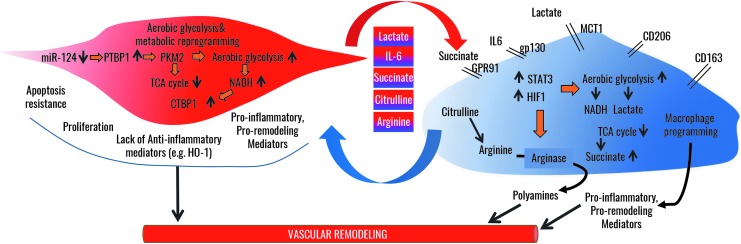
FIG. 6.
Metabolic synergy between adventitial fibroblasts and macrophages in the vascular remodeling process in PH. In adventitial fibroblasts, epigenetic changes reflected in decreased miRNA124 expression enable increased PTBP1 signaling, resulting in increased expression of PKM2, which drives lactate formation and increases aerobic glycolysis while reducing TCA cycle. Increased glycolysis results in increased free NADH, which activates the transcriptional repressor CtBP1. Together, these alterations promote proliferation and apoptosis resistance concomitantly with an increased production of lactate, succinate, citrulline, IL-6, and other pro-inflammatory mediators, while suppressing anti-inflammatory mediator production. Adventitial macrophages respond to the increased concentrations of fibroblast-derived metabolites in the microenvironment whereby IL6, lactate, and succinate drive activation of STAT3 and HIF1, which, in turn, drive metabolic reprogramming similar to that in adventitial fibroblasts; utilization of citrulline feeds polyanine production by Arginase, which, in turn, is increased in response to IL6 and lactate. Thus, exchange of substrates and metabolites between macrophages and fibroblasts enables persistent metabolic reprogramming, cellular activation, and proliferation. PTBP1, polypyrimidine-tract binding protein 1; TCA, tricarboxylic acid.