Abstract
Alzheimer's disease (AD) is the most common neurodegenerative disorder affecting the elderly population worldwide. Brain inflammation plays a key role in the progression of AD. Deposition of senile plaques in the brain stimulates an inflammatory response with the overexpression of pro-inflammatory mediators, such as the neuroinflammatory cytokine. interleukin-6. Curcumin has been revealed to be a potential agent for treating AD following different neuroprotective mechanisms, such as inhibition of aggregation and decrease in brain inflammation. We synthesized new curcumin derivatives with the aim of providing good anti-aggregation capacity but also improved anti-inflammatory activity. Nine curcumin derivatives were synthesized by etherification and esterification of the aromatic region. From these derivatives, compound 8 exhibited an anti-inflammatory effect similar to curcumin, while compounds 3, 4, and 10 were more potent. Moreover, when the anti-aggregation activity is considered, compounds 3, 4, 5, 6, and 10 showed biological activity in vitro. Compound 4 exhibited a strong anti-aggregation effect higher than curcumin. Monofunctionalized curcumin derivatives showed better bioactivity than difunctionalized compounds. Moreover, the presence of bulky groups in the chemical structure of curcumin derivatives decreased bioactivity.
Keywords: Aβ aggregation, anti-inflammatory activity, Alzheimer's disease, brain inflammation, curcumin, curcumin derivatives, IL-6, synthesis
Introduction
Alzheimer's disease (AD) is the most common neurodegenerative condition, affecting more than 29 million people around the world, a number that is expected to triple by 2050. AD neuropathology is characterized by intraneuronal neurofibrillary tangles and extracellular senile plaques in the brain. Neurofibrillary tangles are composed of hyperphosphorylated tau proteins, while senile plaques originate from amyloid-β (Aβ) aggregation [1].
Inflammation is a complex biological response to a harmful stimuli or cell/tissue damage [2, 3]. Chronic brain inflammation is a distinctive feature of AD in which the microglia, astrocytes, and, to a certain extent, neurons are thought to be strongly involved in the inflammatory process. Furthermore, the overexpression of pro-inflammatory mediators, such as tumor necrosis factor -α and interleukin (IL)-6, and acute proteins are evident in different regions of an AD brain [4]. A synergistic pattern between AD senile plaques and pro-inflammatory cytokines increases the neurological damage to the brain [5, 6]. Thus, an increased deposition of Aβ proteins potentiate the production of pro-inflammatory cytokines, while these cytokines promote the formation of other constitutive proteins of the senile plaques.
The strong association between brain inflammation and AD pathology has stimulated research toward the discovery of new therapeutic agents that are likely to provide benefits to patients with AD. In this sense, the anti-inflammatory activity of natural products, which therefore decrease the impact of AD in patients, has been studied.
Curcumin (Fig. 1), a major polyphenol of the rhizome of Curcuma longa, is a potent anti-inflammatory and neuroprotective natural product [1]. Studies in vitro have revealed that curcumin inhibits amyloid β-aggregation, the activities of the enzymes β-secretase and acetylcholinesterase, and Aβ-induced inflammation [7, 8]. In vivo, this polyphenol inhibits Aβ oligomerization, Aβ deposition, and tau phosphorylation in AD animalmodels [7, 8].
Fig.1.
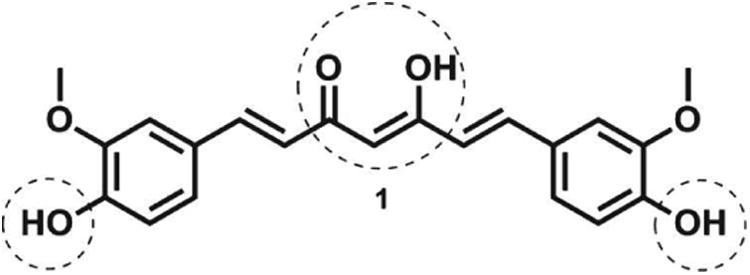
Curcumin and its major reactive sites.
The anti-inflammatory activity of curcumin is mediated by modulation of several molecules involved in the inflammatory process.
In vitro, curcumin inhibits the production of pro-inflammatory cytokines, regulates the activity of inflammatory enzymes (COX-2, and the inducible nitric oxide synthase), and downregulates the expression of chemokines (MCP-1 and interferon-inducible protein) [9]. Meanwhile, in vivo experiments show it regulates the activation of transcription factors such as activating protein-1 and nuclear factor- κB [9]. The lack of toxicity of curcumin at high concentrations makes it a potential nonsteroidal anti-inflammatory drug. Its low bioavailability, due to susceptibility to degradation in biological systems and poor solubility in water and plasma has, however, prevented the medical use of curcumin [11]. Although it is plausible that the anti-inflammatory activity of curcumin can be improved through chemical modification, there have been only few studies on the synthesis of curcumin analogs with this aim [10–13]. Thus, we sought to design and synthesize new anti-inflammatory curcumin derivatives with a higher anti-inflammatory effect than curcumin and good capacity to inhibit Aβ aggregation.
Materials and Methods
Synthesis
Chemical reagents used were commercially available (Tedia, Applichem, Chem-Impex International, Sigma Aldrich, Oakwood Products, Lancaster Avocado, Alfa-Aesar, Fisher). All reactions were conducted with magnetic stirring under an argon atmosphere in oven-dried flasks. Reactions were monitored until deemed complete by TLC using silica-gel-coated glass plates (Merck Kiselgel 60 F254). Plates were visualized under UV light (254nm). Plates were dyed with 10% phosphomolybdic acid (PMA) in ethanol. 1H, and 13C NMR spectra were recorded at 500 (1H), and 125MHz (13C) on an Agilent Inova 500 spectrometer; and at 400 (1H), 100MHz (13C) on Eclipse 400MHz spectrometer (JEOL, Peabody, MA, USA). Chemical shifts (δ) are reported in parts per million (ppm) using the residual solvent peak and coupling constants (J) are given in Hz. Proton multiplicity is reported as singlet (s), doublet (d), triplet (t), quartet (quart.), quintet (quint.), septet (sept), multiplet (m), and broad (br). Infrared spectrophotometry was carried out on a Platinum ATR Alpha instrument (Brucker, Billerica, MA, USA). The molar masses were determined with a micrOTOF-QIII spectrometer Bruker Daltonics, Billerica, MA, USA), with electrospray ionization (ESI) and positive ion detection mode. The detailed synthetic procedures and spectral characterization are described below.
General procedure (GP1) for the synthesis of alkyl succinates S1-S3
An oven-dried round bottom flask was charged with the given alcohol (14.7 mmol), dichloromethane (5 mL), and N, N-diisopropylethylamine (1.3 mL, 7.35 mmol, 0.5 equiv.) at room temperature (RT). After 2 h, succinic anhydride (735 mg, 7.35 mmol, 0.5 equiv.), and 4-dimethylaminopyridine (448 mg, 3.67 mmol, 0.25 equiv.) were added, and the reaction stirred at RT. After 48 h, the reaction mixture was diluted with brine/1 M HCl (3:1, 10 mL). The aqueous layer was extracted with dichloromethane (3×10 mL). The combined organic phases were dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure. The crude material was washed with hexanes (3×20 mL) to obtain the desired product.
Synthesis of 4-(allyloxy)-4-oxobutanoic acid (S1)
According to GP1, allyl alcohol (854 mg, 14.7 mmol) yielded S1 (790 mg, 40%).
Synthesis of 4-(benzyloxy)-4-oxobutanoic acid(S2)
According to GP1, benzyl alcohol (3 g, 27.7 mmol, 2.88 mL), pyridine (20 mL), 4-dimethy-laminopyridine (508 mg, 4.15 mmol, 0.15 equiv.), and succinic anhydride (1.3 g, 13.8 mmol, 0.5 equiv.) were combined, and the reaction was stirred for 6 h at 100C to yield S2 (3.74 g, 65%).
Synthesis of 4-(cyclopentyloxy)-4-oxobutanoic acid (S3)
According to GP1, cyclopentanol (949 mg, 11 mmol, 1 mL) and N,N-diisopropylethylamine (958 μL, 5.5 mmol, 0.5 equiv.) were stirred in dichloromethane (5 mL) at RT. After 2 h, succinic anhydride (550 mg, 5.5 mmol, 0.5 equiv.) and 4-dimethylaminopyridine (672 mg, 5.5 mmol, 0.5 equiv.) were added and the reaction stirred for 48 h at RT to yield S3 (1 g, 50 %).
General procedure (GP2) for the synthesis of dialkylcurcumin and monoalkylcurcumin (5-10)
An oven-dried round bottom flask, equipped with magnetic stirrer and 3 Å molecular sieves, was flushed with argon and charged with alkyl succinate (207 mg, 1.3 mmol, 10 equiv.), pyridine (3 mL), 4-dimethylaminopyridine (366 mg, 0.39 mmol, 3 equiv.), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (75 mg, 0.39 mmol, 3 equiv.). The reaction was stirred for 4 h at RT. Concurrently, a solution of curcumin (1) (50 mg, 0.13 mmol) in pyridine (3 mL) was stirred for 4 h at RT. The curcumin solution was then added to the alkyl succinate reaction allowed to stir for 48 h at RT. The reaction mixture was diluted with a 0.5 M aqueous solution of Na2CO3/brine (1:1, 10 mL), and the aqueous layer extracted with ethyl acetate (EtOAc) (3×10 mL). The combined organic phases were dried over Na2SO4, filtered, concentrated under reduced pressure, and purified by preparative HPLC using normal phase silica gel column (Phenomenex, Sphereclone, 250×10mm, 5μm) with an n-hexane to ethyl acetate gradient system in 20 min at 2 mL/min to obtain the desired products.
Synthesis of diallyl O,O′-(((1E,3Z,6E)-3-hydroxy-5-oxohepta-1,3,6-triene-1,7-diyl)bis(2-methoxy-4,1-phenylene)) disuccinate (5), and allyl (4-((1E,4Z,6E)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-3-oxohepta-1,4,6-trien-1-yl)-2-methoxyphenyl) succinate (6)
According to GP2, S1 (207 mg, 0.67 mmol, 10 equiv), pyridine (3 mL), 4-dimethylaminopyridine (366 mg, 0.39 mmol, 3 equiv.), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (75 mg, 0.39 mmol, 3 equiv.) were combined and stirred at RT. Concurrently, a solution of curcumin (50 mg, 0.13 mmol) in pyridine (3 mL) was stirred at RT. After 4 h, the reactions were combined and allowed to stir for 48 h at RT. The crude product was purified by HPLC with diode array UV detection (DAD) to obtain 5 (19 mg, 22%) and 6 (27 mg, 39%).
Synthesis of dibenzyl O,O′-(((1E,3Z,6E)-3-hydroxy-5-oxohepta-1,3,6triene-1,7-diyl)bis(2-methoxy-4,1-phenylene)) disuccinate (7 ), and benzyl (4-((1E,4Z,6E)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-3-oxohepta-1,4,6-trien-1-yl)-2-methoxyphenyl) succinate (8)
According to GP2,S2 (64 mg, 1.3 mmol, 10 equiv.), pyridine (3 mL),4-dimethylaminopyridine (366 mg, 0.39 mmol,3 equiv.), and 1-ethyl-3-(3-dimethylaminopro-pyl)carbodiimide hydrochloride (75 mg, 0.39 mmol, 3 equiv.) were stirred at RT. Concurrently, a solution of curcumin (50 mg, 0.13 mmol) in pyridine (3 mL) was stirred at RT. After 4 h, the solutions were combined and allowed to stir for 48 h at RT. The crude product was purified by HPLC-DAD to obtain 7 (21 mg, 21%) and 8 (28 mg, 37%).
Synthesis of dicyclopentyl O,O′-(((1E,3Z,6E)-3-hydroxy-5-oxohepta-1,3,6-triene-1,7-diyl)bis(2-methoxy-4,1-phenylene)) disuccinate (9), and cyclopentyl (4-((1E,4Z,6E)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-3-oxohepta-1,4,6-trien-1-yl)-2-methoxyphenyl) succinate (10)
According to GP2,S3 (245 mg, 1.3 mmol, 10 equiv.), pyridine (3 mL),4-dimethylaminopyridine (366 mg, 0.39 mmol, 3equiv.), and 1-ethyl-3-(3-dimethylamino-propyl)carbodiimide hydrochloride (75 mg, 0.39 mmol, 3 equiv.) was stirred at RT. Concurrently, a solution of curcumin (50 mg, 0.13 mmol) in pyridine (3 mL) was stirred at RT. After 4 h, the solutions were combined and allowed to stir for 48 h at RT. The crude product was purified by HPLC-DAD to obtain 9 (17 mg, 18%) and 10 (25 mg, 34%).
General procedure (GP3) for the synthesis of etherification and estherification of curcumin (2–4)
An oven dried round bottom flask equipped with magnetic stirrer and 3 Å molecular sieves was flushed with argon and charged with curcumin (200 mg, 0.54 mmol), solvent (6 mL), base (150 mg, 1.08 mmol, 2 equiv.), and alkyl halide (300 μL, 2.7 mmol, 5 equiv.). The reaction was stirred for 48 h at RT. The reaction mixture was diluted with water and extracted with EtOAc (3×10 mL). The combined organic phases were dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was washed with n-hexane (3×20 mL) to obtain the desired product.
Synthesis of (1E,6E)-1,7-bis(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)hepta-1,6-diene-3,5-dione (2)
According to GP3, curcumin (200 mg, 0.54 mmol), N,N-dimethylformamide (6 mL), K2CO3 (150 mg, 1.08 mmol, 2 equiv.), and propargyl bromide (300 μL, 2.7 mmol, 5 equiv.) were stirred for 48 h at RT. The reaction mixture was diluted with water (10 mL) and extracted with EtOAc (3 ×10 mL). The combined organic phases were dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was washed with n-hexane (3×20 mL) to yield 2 (168 mg, 70%).
Synthesis of (1E, 4Z, 6E)-1-(4-(benzyloxy)-3-methoxyphenyl)-5-hydroxy-7-(4-hydroxy-3-metho-xyphenyl)hepta-1,4,6-trien-3-one (3)
According to GP3, curcumin (100 mg, 0.27 mmol), acetone (5 mL), Cs2CO3 (88 mg, 0.27 mmol, 1 equiv.), and benzyl bromide (1 mL, 8.1 mmol, 30 equiv.) were stirred at 56°C for 24 h. The reaction mixture was diluted with water (20 mL) and extracted with EtOAc (3×10 mL). The combined organic phases were dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography [n-hexane/EtOAc, silica gel] to obtain 3(98 mg, 80%).
Synthesis of 4-((1E,4Z,6E)-5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-3-oxohepta-1,4,6-trien-1-yl)-2-methoxyphenyl acetate (4)
According to GP3, curcumin (100 mg, 0.27 mmol) and K2CO3 (38 mg, 0.27 mmol, 1 equiv.) in dichloromethane (10 mL) were stirred at RT. After 8 h, a solution of acetyl chloride (96μL, 1.35 mmol, 5 equiv.) in dichloromethane (5 mL) was sonicated for 5 min and added to the curcumin solution. The reaction was stirred for 24 h at RT followed by column chromatography [n-hexane/EtOAc, silica gel] to obtain 4 (79 mg, 71%).
A complete detail of the methods used for synthesis and for the characterization of the synthesized compounds can be found in the Supplementary material.
Mice
Female C57Bl/6 mice, 8 weeks of age, were provided by INDICASAT's animal facility. Animals were maintained in 12 h light/dark cycle at a constant temperature of 24°C with free access to food and water. Experimental procedures were performed following the ethical guidelines related to the handling of lab animals in accordance with international and institutional regulations. The Institutional Animal Care and Use Committee of INDICASAT approved the protocol (IACUC-15-004).
Cell culture and cytokine determination
To determine the anti-inflammatory capacity of compounds (1–6, 8, 10), elicited peritoneal macrophages from C57Bl/6 mice were obtained by peritoneal washing with chilled RPMI after 5 days of i.p. instillation of 2 mL of 3% thioglycollate. Cells were seeded in RPMI with 10% FCS at 2×105 cells/well in 96-well plates and incubated at 37°C in a 5% CO2 atmosphere. Cells were stimulated with LPS (10 ng/mL) in the presence or absence of different concentrations of compounds (1, 3, 10, and 30μM). All the treatments and controls were performed in the presence of 0.5% DMSO, the vehicle for test compounds. Supernatants were collected 18 h after stimulation with LPS, and the concentrations of IL-6 were determined by ELISA. Concentrations of cytokine were measured following the manufacturer's protocol (DuoSet kit, R&D System).
Cytotoxicity assay
To determine the cytotoxicity of the compounds tested (1–6, 8, 10), 100μL of MTT at a concentration of 0.5 mg/mL in RPMI was added to each well after removing the supernatant and were incubated for 2 h at 37°C in 5% CO2. MTT (soluble tetrazolium salt) is reduced to formazan (insoluble) by the activity of succinate dehydrogenase of living cells' mitochondria. The supernatants were removed, and the formazan crystals were dissolved in 100μL of 0.04 M HCl in isopropanol. The color was analyzed at 570 nm using a plate reader. The percentage of viable cells was calculated as % viability=(OD sample/OD control)× 100%. Non-stimulated cells cultured in media represent 100% viability.
Thioflavin T assay
The aggregation of Aβ42 was evaluated by the thioflavin T assay. The rAβ42 (rPeptide) was resuspended according to the manufacturer's instruction in 1% NH4OH, at a concentration of 1 mg/ml after 1 min of hydration. rAβ42 (10μM) was combined with or without different concentrations (1, 3, 10, and 30μM) of compounds, and 200μL of the mix was plated in a 96-well black plate. For this assay, curcumin (5μM) was used as inhibition control. All of the reactions were performed in the presence of 0.1% of DMSO of the vehicle for the compounds. The plate was incubated at 37C for 48 h. After incubation, 20μM of thioflavin T was added and the fluorescence was measured in a Synergy HT multi-reader from Biotek (Winooski, VT), excitation 450 nm, emission 485 nm. Fluorescence values were determined by subtracting the baseline fluorescence of thioflavin T.
Statistical analysis
Data were analyzed by using the statistical software package GraphPad Prism5. Statistical analysis was performed with the unpaired t test. A significant difference between groups was considered to be when p<0.05. The half maximal inhibitory concentration (IC50) was calculated by adjusting a sigmoidal dose-response curve following the procedure in GraphPad Prism5.
Results
Curcumin derivatives
In this study, seven novel curcumin derivatives (3, 5, 6, 7, 8, 10) were synthesized. Moreover, two known compounds (2, 4) were also synthesized with the purpose of establishing a structure-activity relationship (SAR).
Compounds 2-3 were synthesized by etherification reactions, while compounds 4–10 were formed by esterification (Fig. 2). The reaction between curcumin and propargyl bromide in the presence of K2CO3 and DMF afforded 2 in 70% yield. Curcumin and benzyl bromide reacted in the presence of Cs2CO3 and DMF to afford 3 in 80% yield. The reaction between curcumin and acetyl chloride in the presence of K2CO3 and acetone produced 4 in 71% yield (Fig. 2).
Fig.2.
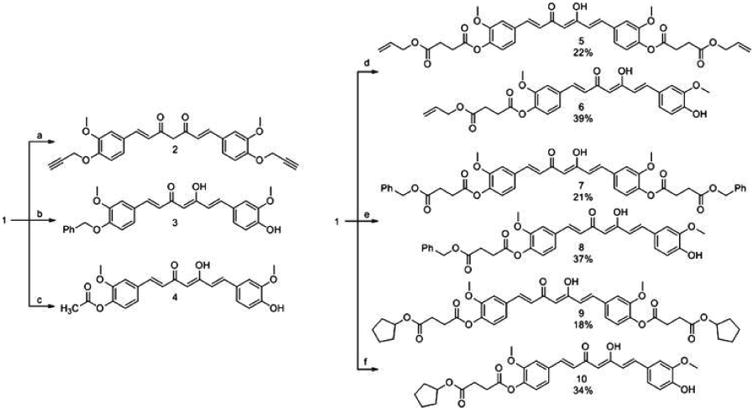
Synthesis of curcumin derivatives with ether, ester, and diesther groups. Reagents and conditions: a) CHCCH2Br, K2CO3, DMF, RT, 48 h, 70%. b) C6H5CH2Br, Cs2CO3, CH3COCH3, 56C, 24 h, 80%. c) CH3COCl, K2CO3, CH2Cl2, RT, 24 h, 71%. d) S1, DMAP, EDC, pyridine, RT, 48 h, 22% (5), and 39% (6). e) S2, DMAP, EDC, pyridine, RT, 48 h, 21% (7), and 37% (8). f) S3, DMAP, EDC, pyridine, RT, 48 h, 18% (9), and 34% (10).
The reaction between curcumin and 4-(allyloxy)-4-oxobutanoic acid in the presence of EDC, DMAP, and pyridine produced difunctionalized (5, 22%), and monofunctionalized (6, 39%) succinate analogs (Fig. 2). On the other hand, when curcumin reacted with 4-(benzyloxy)-4-oxobutanoic acid in the presence of EDC, DMAP, and pyridine, difunctionalized ester 7 and monofunctionalized ester 8 derivatives were produced in 21% and 37% yield, respectively. Finally, when curcumin reacted with 4-(cyclopentyloxy)-4-oxobutanoic acid in the presence of EDC, DMAP, and pyridine, esterified analogs 9 and 10 were produced in 18% and 34% yield, respectively.
Anti-inflammatory activity of curcumin derivatives
To evaluate the anti-inflammatory activity of the curcumin analogs and curcumin, we measured the secretion of IL-6 by murine macrophages stimulated with LPS in the presence or absence of the compounds. Compounds 2–6, 8, and 10 reduced macrophage response, while compounds 7 and 9 did not show any anti-inflammatory activity. Compounds 2–4 completely suppressed the production of IL-6 at 10μM (Fig. 3a). This inhibition was not due to a cytotoxic effect of the compounds, since cell viability was not affected at the evaluated concentrations (Fig. 3b). Compounds 5, 6, 8, and 10 showed mid-to-strong inhibition of IL-6 production (Fig. 3c), with a complete inhibition of IL-6 at 30μM. Compounds 6, 8, and 10 did not affect the cell viability at the concentrations tested (Fig. 3d). Compound 5 exhibited cytotoxicity at a concentration of 30μM. The IC50 values for curcumin and its analogs are shown in Table 1.
Fig.3.
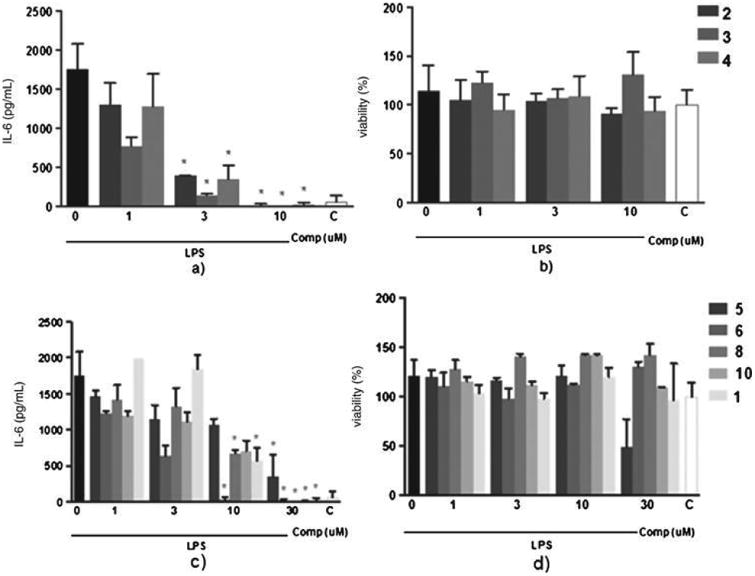
Anti-inflammatory activity of curcumin derivatives with ether, ester, and diester groups. Peritoneal macrophages from C57BI/6 mice were treated with different concentrations (1, 3, 10, or 30μM) of the evaluated compounds 1 h before the stimulus with 10 ng/mL of LPS. After 24 h, concentration of IL-6 was determined by the ELISA method in the supernatant of cells treated with curcumin derivatives with ether and ester groups (a) or with diester groups (c). Cells viability was assessed by the MTT assay after supernatant collection (b and d). All results are represented as Mean±S.E.M from three independent experiments performed in duplicate. *p<0.05 relative to the LPS stimulus alone. C, negative control.
Table 1. Chemical structures and anti-inflammatory and anti-amyloid aggregation activity of synthetic curcumin derivatives.
| Compound Structure | aIC50±S.D (μM) | bThT assay IC50 (μM) | |
|---|---|---|---|
| 2 |
|
2.23±0.84 | NA |
| 3 |
|
1.81±1.31 | 2.05±0.1 |
| 4 |
|
2.21±0.93 | 1.32±0.7 |
| 5 |
|
14.2±12.8 | 1.96±0.7 |
| 6 |
|
2.50±0.92 | 1.77±0.9 |
| 8 |
|
8.28±3.08 | NA |
| 10 |
|
3.22±1.34 | 2.04±0.7 |
| 1 |
|
8.25±1.25 | 1.49±0.9 |
Values represent average of IC50 from three independent experiments performed in duplicate±S.D.
Curcumin derivatives decrease Aβ aggregation
To evaluate the effect of curcumin derivatives on Aβ aggregation, Aβ1 - 42 was incubated for 48 h in the presence or absence of compounds. Fibrils of Aβ were detected by a thioflavin T assay. Compounds 3, 4, 5, 6, and 10 inhibited the aggregation of Aβ in a concentration-dependent manner (Fig. 4) with IC50 values ranging from 1.32±0.7μM to 2.05±0.1μM (Table 1). These compounds showed a similar effect on amyloid aggregation as compared to curcumin (1) (IC50=1.4±0.9). Compounds 2, 8, and 9 did not present anti-aggregation activity.
Fig.4.
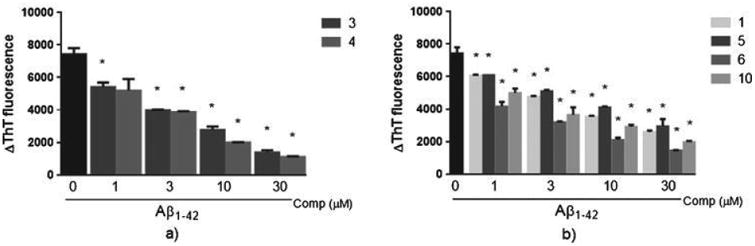
Effect of curcumin derivatives with ether, ester, and diester groups on Aβ aggregation. Aβ42 recombinant peptide was incubated during 48 h with or without different concentrations (1, 3, 10, or 30μM) of compounds. After incubation, ThT (20μM) was added and was determined.changes in its fluorescence intensity (ΔThT fluorescence) in the presence of curcumin derivatives with ether and ester groups (a) or with diester groups (b). Results are represented as Mean±S.D. from three independent experiments performed in duplicate. *p<0.05 relative to Aβ42 alone.
Discussion
New curcumin derivatives were prepared by etherification and esterification in order to provide structural changes that could potentially increase anti-inflammatory activity. Inhibition of the secretion of pro-inflammatory cytokines, such as IL-6, is frequently used as readout of an anti-inflammatory activity. We evaluated the effect of curcumin derivatives on the production of IL-6 by macrophages stimulated with LPS. Assessment of the bioactivity in vitro showed that compounds 2–6, 8, and 10 decreased the secretion of IL-6, depending on the chemical modification. Compounds 2–4 down regulated the production of IL-6 in a concentration-dependent manner, with a negligible release at 10μM. A structure-activity relationship of curcumin derivatives was investigated by introducing changes on the hydroxyl groups located on the aromatic rings and evaluating the anti-inflammatory activity. Curcumin modified with small groups by etherification of the hydroxyl groups on both aromatic rings (2) showed a much higher anti-inflammatory activity than did unmodified curcumin (2.23±0.84 versus 8.25±1.25) (Table 1). Furthermore, introduction of a benzene ring etherified at one of the curcumin rings led to a curcumin derivative with the most potent anti-inflammatory activity (3). Acetylation at only one side of the molecule resulted in strong biological activity. However, when the complexity and length of the groups attached to both rings increased, a reduced (5), or null biological activity (7, 9), occurred as compared to curcumin. Indeed, a strong improvement on the activity was achieved when modifications were done at only one of the aromatic rings (6, 8, 10). When monofunctionalized diester curcumin derivatives are considered (6, 8, 10), it was observed that the improvement of the anti-inflammatory activity was reduced by the presence of bulky groups in the molecule. These bulky groups may disturb the molecular mechanisms by which these derivatives inhibit the production of IL-6 in a fashion that needs further investigation.
Based on the results obtained from the anti-inflammatory activity of curcumin and its analogues, it can be concluded that hydroxyl groups on the aromatic rings of the curcumin are pharmacophores, required for reducing the production of IL-6. Moreover, modifications on curcumin to produce new analogs with potential anti-inflammatory activity by inhibition of IL-6 should take into account the following salient points (1) that at least one hydroxyl group of the aromatic rings should not be modified, and (2) etherification and esterification of only one of the hydroxyl groups present in the benzene rings will strongly enhance the activity depending on the complexity of the substituent added.
At the molecular level, AD is characterized by the presence of extracellular Aβ senile plaques in the brain [1, 5]. Senile plaques are produced by the cleavage of the amyloid-β protein precursor (AβPP) by the enzyme β-secretase at the AβPP beta site, leading to a small soluble AβPPβ fragment and a C-99 fragment of AβPP. The C-99 fragment is then broken by the enzyme γ-secretase into two fragments of AβPP intracellular domain protein and the pathological Aβ42 peptide, which polymerizes forming amyloid fibrils, leading to cell death in the brain [1].
The effect of curcumin on Aβ aggregation has been extensively studied [14, 15]. This effect appears to be favoring the generation of non-toxic Aβ intermediates during the formation of fibrils [16]. It has also been proposed that curcumin disaggregates Aβ fibrils [17]. Compounds 3, 4, 5, 6 and 10 presented anti-aggregation activity in vitro (Table 1). SAR evaluation indicates that curcumin derivatives etherified with small groups at both sides of the molecule lose the anti-aggregation activity (2). Nevertheless, etherification at only one side of curcumin maintains the bioactivity, yet is lower than curcumin (3). Acetylation at only one side of the molecule led to an analog with a larger biological activity (4). Monofuctionalized diester analogs (6, 8, 10) showed a decreased or null bioactivity compared to curcumin. This decrease in the anti-aggregation activity is associated with the complexity and length of the analog. The presence of bulky groups in monofuctionalized diester derivatives impacted negatively the activity. When bifunctional diester is taken into account (5), a reduced biological activity was also observed. It has been suggested that phenolic compounds, such as curcumin, are able to produce anti-aggregation activity due to π - π interactions and the formation of hydrogen bonds between the peptide and the phenolic rings [18]. These anti-aggregation mechanisms might be also followed by compounds 3, 4, 5, 6, and 10, yet the exact mechanism needs to be studied. Since we measured the anti-aggregation activity of the compounds in a time period of 48 h, we cannot rule out the possibility that these curcumin derivatives might have disaggregation potential. Kinetics studies are necessary to evaluate a potential disaggregate effect of these new curcumin derivatives.
Inhibition of Aβ aggregation is a promising approach for the identification of new agents for AD treatment. Several synthetic and natural compounds have been tested as inhibitors for Aβ aggregation [1]. Some molecules have reached different phases of clinical trials; however, until now, no molecule has been approved as new therapeutic. Hence, the search of new molecules with anti-aggregation effect continues to be of great interest.
Our findings suggest that the novel curcumin derivatives 3, 4, 6, and 10 have potential as therapeutic compounds for the treatment of AD.
Supplementary Material
Acknowledgments
AAA-D greatly acknowledges financial support from SENACYT (ECS11-002) and INDICASAT-BID (02-12). Financial support to OVL by the Welch Foundation (AX-1788), NIGMS (SC3GM105579), UTSA, and the NSF (CHE-1455061) is gratefully acknowledged. KSR, MG, EM, PLF, and AAD-A would like to acknowledge SENACYT (Panama) and the National System of Investigators (SNI) for supporting their research. JL-B and YG are supported by the Institute for training and Development of Human Resources (IFARHU), and SENACYT (Panama). JL-B is also supported by the Ministry of Economy and Finance (DIPRENA-DPIP-10866-2013) on Nutritive Supplements, and by Melo Brain Grant (Panama). Mass spectroscopic analysis was supported by a grant from the National Institute on Minority Health and Health Disparities (G12MD007591). Authors want to acknowledge Michell Moran and Sahyra Marin for technical collaboration.
Footnotes
Authors' disclosures available online (http://j-alz.com/manuscript-disclosures/17-0071r2).
Appendices: The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170071.
References
- 1.Lakey-Beitia J, Berrocal R, Rao KS, Durant AA. Polyphenols as therapeutic molecules in Alzheimer's disease through modulating amyloid pathways. Mol Neurobiol. 2015;51:466–479. doi: 10.1007/s12035-014-8722-9. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.González Y, Torres-Mendoza D, Jones GE, Fernandez PL. Marine diterpenoids as potential anti-inflammatory agents. Mediators Inflamm. 2015;2015:263543. doi: 10.1155/2015/263543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crispoltoni L, Stabile AM, Pistilli A, Venturelli M, Cerulli G, Fonte C, Smania N, Schena F, Rende M. Changes in plasma β-NGF and its receptors expression on peripheral blood monocytes during Alzheimer's disease progression. J Alzheimers Dis. 2017;55:1005–1017. doi: 10.3233/JAD-160625. [DOI] [PubMed] [Google Scholar]
- 5.Rubio-Perez JM, Morillas-Ruiz JM. A review: Inflammatory process in Alzheimer's disease, role of cytokines. Scientific World Journal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative 6. diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamaguchi T, Ono K, Yamada M. Review: Curcumin and Alzheimer's disease. CNS Neurosci Ther. 2010;16:285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao EC, Xue L. Therapeutic effects of curcumin on Alzheimer's disease. Adv Alzheimer Dis. 2014;3:145–159. [Google Scholar]
- 9.Chainani-Wu N. Safety and anti-inflammatory activity of curcum: A comonent of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 10.ZY Du, Wei X, Huang MT, Zheng X, Liu Y, Conney AH, Zhang K. Anti-proliferative, anti-inflammatory and antioxidant effects of curcumin analogue A2. Arch Pharm Res. 2013;36:1204–1210. doi: 10.1007/s12272-013-0216-1. [DOI] [PubMed] [Google Scholar]
- 11.Wu J, Zhang Y, Cai Y, Wang J, Weng B, Tang Q, Chen X, Pan Z, Liang G, Yang S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg Med Chem. 2013;21:3058–3065. doi: 10.1016/j.bmc.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Li Y, Yue Y, Zhang K, Chen Q, Wang H, Lu Y, Huang MT, Zheng X, Du Z. Synthesis and biological evaluation of curcumin derivatives containing NSAIDs for their anti-inflammatory activity. Bioorg Med Chem Lett. 2015;25:3044–3051. doi: 10.1016/j.bmcl.2015.04.077. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Zhang Y, Zou P, Wang J, He W, Shi D, Li H, Liang G, Yang S. Synthesis and biological evaluation of a novel class of curcumin analogs as anti-inflammatory agents for prevention and treatment of sepsis in mouse model. Drug Des Devel Ther. 2015;18:1663–1678. doi: 10.2147/DDDT.S75862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thapa A, Jett SD, Chi EY. Curcumin attenuatesamyloid-β aggregate toxicity andmodulates amyloid-β aggregationpathway. ACS Chem Neurosci. 2016;7:56–68. doi: 10.1021/acschemneuro.5b00214. [DOI] [PubMed] [Google Scholar]
- 15.Potter PE. Curcumin: A natural substance withotential efficacy in Alzheimer's disease. J Exp Pharmacol. 2013;5:23–31. doi: 10.2147/JEP.S26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao PP, Mohamed T, Teckwani K, Tin G. Curcumin binding to beta amyloid: A computational study. Chem Biol Drug Des. 2015;86:813–820. doi: 10.1111/cbdd.12552. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 18.Dolai S, Shi W, Corbo C, Sun C, Averick S, Obeysekera D, Farid M, Alonso A, Banerjee P, Raja K. “Clicked” sugar-curcumin conjugate: Modulator of amyloid-β and tau peptide aggregation at ultralow concentrations. ACS Chem Neurosci. 2011;2:694. doi: 10.1021/cn200088r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


