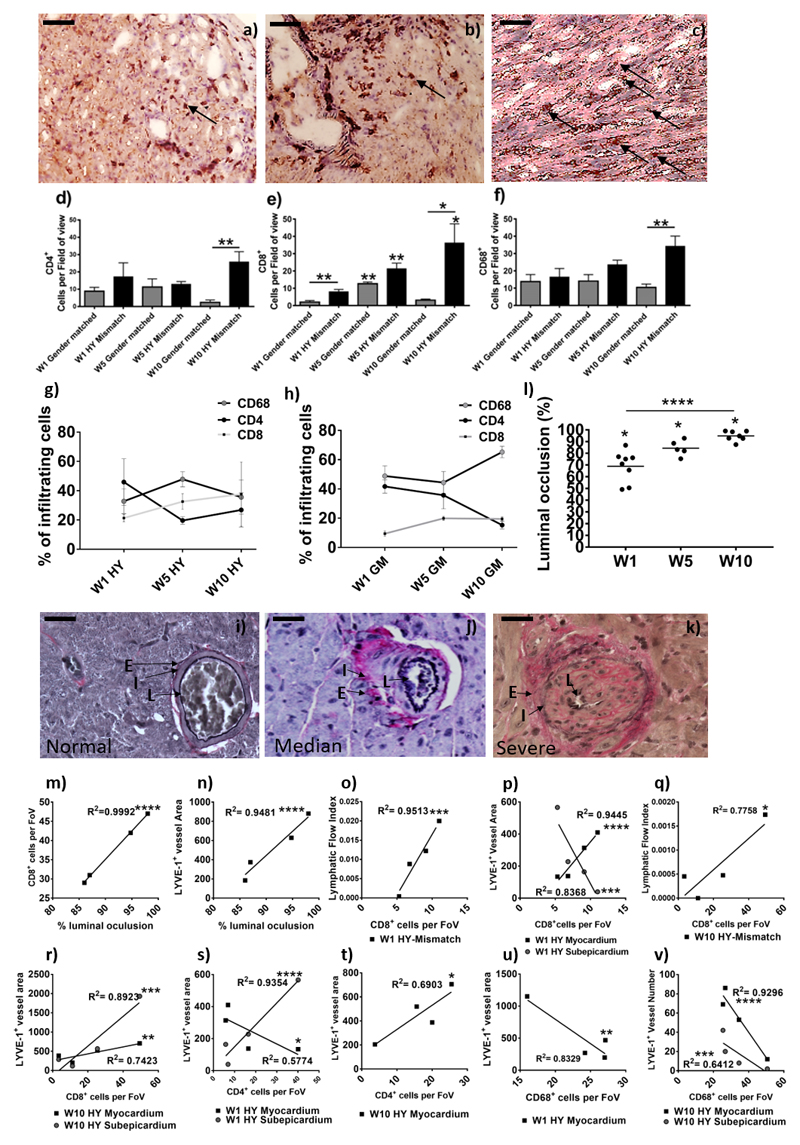
Figure 4. Determination of graft vasculopathy and inflammatory cellular infiltration within the transplanted donor grafts.
After SPECT/CT scanning donor grafts were harvested, immunohistochemical staining for CD4 (a) CD8 (b), and CD68 (c) were conducted to determine cell numbers (black arrows show an example); shown is a representative of HY-mismatched grafts at ten weeks post-transplant. Images are representative examples of 4 independent experiments; scale bars = 50 μm. Positive CD4 (d, **p = 0.0079) and CD8 (e, W5GM**p =0.0019, W5HY**p = 0.0066, W10HY**p = 0.0429, W1GM vs. HY**p = 0.0077, W10GM vs. HY*p = 0.0233) cells were counted in 20 random high-power fields (magnification 400x) by two independent observers. For CD68+ macrophage staining (f, W10**p = 0.0079), a 63-point grid was overlaid onto a 400x magnification of macrophage staining. The percentages of grid points positive for CD68 staining were counted by two independent observers. Data are shown as the mean number of cells, per field of view (FoV), of the two independent observations and four independent experiments ± SEM; of a gender-matched and HY-mismatched grafts at weeks, one, five and ten. Within the infiltrating cells, the percentage of CD68+, CD4+ and CD8+ cells was determined in the HY-mismatched (g) and gender-matched grafts (h). Immunohistochemical staining for Elastic Van Gieson was performed to determine graft vasculopathy. Shown is a representative of a gender-matched (i) and HY-mismatched grafts (j & k). Histologically the allograft vasculopathy ranged from median (j) to severe (k) in the HY mismatched graft. Characterised by inflammatory cell invasion into the layers of the internal to external elastic; shown is the lumen of the vessel (L), the internal elastic (I) and the external elastic (E). Measurement of the internal elastic (I) and the external elastic (E) can be used to calculate the percentage of luminal occlusion (l, W1*p=0.0121, W5*p=0.0357 & W10*p=0.0238; ****p<0.0001). Shown is the percentage luminal occlusion in four independent experiments with mean at one, five and ten weeks. The percentage luminal occlusion was correlated with CD8+ cell number (m, ****p<0.0001) and the mean lymphatic vessel area (n, ****p<0.0001). CD8+ cell number was correlated with the LFI (o, W1****p<0.0001 & q, W10*p=0.0218) and the lymphatic vessel area (p, myocardial W1****p<0.0001, subepicardial W1***p=0.0008 & r, myocardial W10**p=0.006, subepicardial W10***p=0.0004). CD4+ cell number was correlated with the lymphatic vessel area (s, myocardial W1*p=0.0287 & subepicardial W1****p<0.0001 & t, W10*p=0.0206). CD68+ cell number was correlated with the lymphatic vessel area (u, W1**p=0.0016) and density (v, myocardial W10****p<0.0001 & subepicardial W10***p=0.0008).