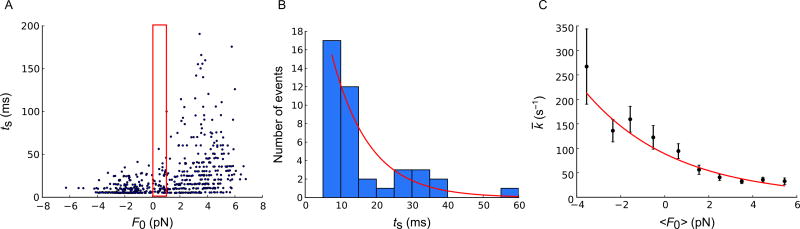
Fig. 4.
Load-dependent ADP release rate measured with 200-Hz stage oscillation. (A) Scatter plot of duration of binding (ts) vs load (F0) (N = 388). (B) Histogram of durations ts in the narrow range of loads in the red box in panel (A) (N = 41). Histogram bins are 5 ms wide, i.e., one period of the stage motion. The histogram is fitted by Eq. (4) using maximum-likelihood estimation (MLE) to obtain k̄ = k(〈F0〉, ΔF).The histogram is consistent with an exponential distribution (full line). The characteristic time of this exponential is the inverse of the ADP release rate under the average load in the red box in panel (A), k̄ = k(〈F0〉, ΔF). (C) ADP release rates depend exponentially on applied load. Black data points show ADP release rates k̄ = k(〈F0〉),ΔF) against mean load 〈F0〉 for each of 10 consecutive 1-pN bins. The individual error bars are calculated from the variance of the MLE in (B) as the inverse Fisher information for the parameter (Sung et al., 2015). The full curve is the fit of Eq. (8) to the rates, yielding k0 = 71 ± 4s−1, δ = 1.01 ±0.09nm, corresponding to k(0, ΔF) = 89s−1 on the full curve. Figure and legend are adapted from Sung, J., Nag, S., Mortensen, K. I., Vestergaard, C. L, Sutton, S., Ruppel, K. M., … Spudich, J. A. (2015). Harmonic force spectroscopy measures load-dependent kinetics of individual human β-cardiac myosin molecules. Nature Communications, 6, 7931. http://dx.doi.org/10.1038/ncomms8931, with a Creative Commons license.