Figure 1.
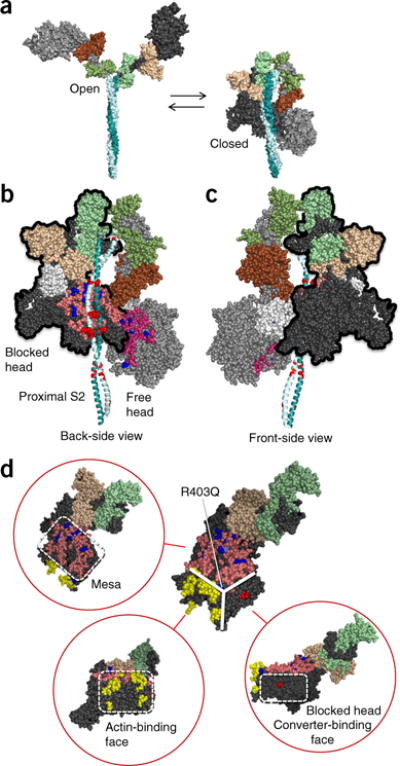
Structural model of sequestered heads of human β-cardiac myosin. The model is based on the 3D reconstruction of tarantula skeletal myosin thick filaments (PDB 3DTP)2. (a) A short version of myosin HMM, showing the first 123 residues of the coiled-coil S2 domain, is illustrated in its open and closed states, which are in equilibrium. The templates used to model the poststroke structure were obtained from the human β-cardiac myosin motor domain45 supplemented with the rigor structure from the squid myosin motor domain63 (Online Methods). (b) The back-side view (side facing the myosin bipolar thick filament) of the sequestered state, showing the potential interaction between different domains of the two heads. The heavy-chain residues of the S1 head on the left (blocked head, outlined in black) are colored pink (mesa residues), dark blue (arginine HCM mutations), light blue (a histidine HCM mutation), white (the converter domain), and dark gray (all remaining residues). The ELC is colored light brown, and the RLC is light green. The heavy-chain residues of the S1 head on the right (free head) are colored dark pink (mesa residues), dark blue (arginine HCM mutations), light blue (a histidine HCM mutation), and light gray (all remaining residues). The ELC is colored dark brown, and the RLC is colored dark green. The proximal S2 tail is shown in teal, with glutamate and aspartate residues mutated in HCM in red. (c) The front-side view of the sequestered state showing the blocked head (outlined in black) interacting with the converter domain (white) of the free head (left). (d) The catalytic domain of human β-cardiac myosin forms a pyramidal structure (thick white solid lines). The three surfaces are the mesa (pink residues with arginine HCM mutations shown in dark blue), the actin-binding interface (yellow, according to the residues involved, as discussed64–67), and the blocked-head surface (dark gray) that abuts the converter domain of the free head in the folded, sequestered-head state (marked by the red residue Asp382, whose mutation to tyrosine is an early-onset HCM mutation). These different interfaces are shown in different orientations of the myosin molecule in the red circles. Coordinates of the homology modeled folded-back human β-cardiac myosin are available for download (MS01, Supplementary Data Set 1).
