Figure 8.
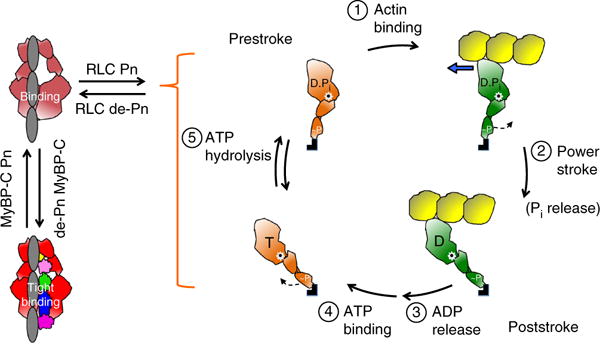
Schematic drawings of the actin-myosin chemomechanical cycle and hypothesized sequestered states of myosin heads. Pn, phosphorylation. (1) The prestroke S1 (orange) with bound ADP (D) and phosphate (Pi) binds to actin (yellow). (2) While bound to actin (green head), the lever arm swings to the right about a fulcrum point (black dot on white star) to the poststroke position, thus moving the actin filament to the left (blue arrow) with respect to the myosin thick filament. (3) ADP release frees the active site for binding of ATP (T). (4) ATP binding weakens the interaction of S1 with actin and frees the lever arm so that it can cock into the prestroke state. (5) ATP hydrolysis locks the head into the prestroke state. The heads in the cycle are phosphorylated (~P) on the RLC. Heads that are sequestered into a nonfunctional state are shown in two states on the left side of the figure: RLC nonphosphorylated and bound to the S2 tail (light red) and complexed with nonphosphorylated MyBP-C and more firmly locked into the sequestered state (bright red). Other than the interactions shown here, many other interactions, for example those involving the LMM and titin, are probably involved in the sequestered state of myosin, and a common theme for HCM mutations may be that they shift the equilibrium away from the sequestered folded-back state of the myosin heads to the open state, which is functionally accessible for interaction with actin, thus producing the hypercontractility observed clinically.
